Method for preparing nano indium oxide with controllable appearance by hydrothermal method
A nano-indium oxide and hydrothermal technology are applied in chemical instruments and methods, inorganic chemistry, gallium/indium/thallium compounds, etc., to achieve the effects of low cost, optimized production process, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
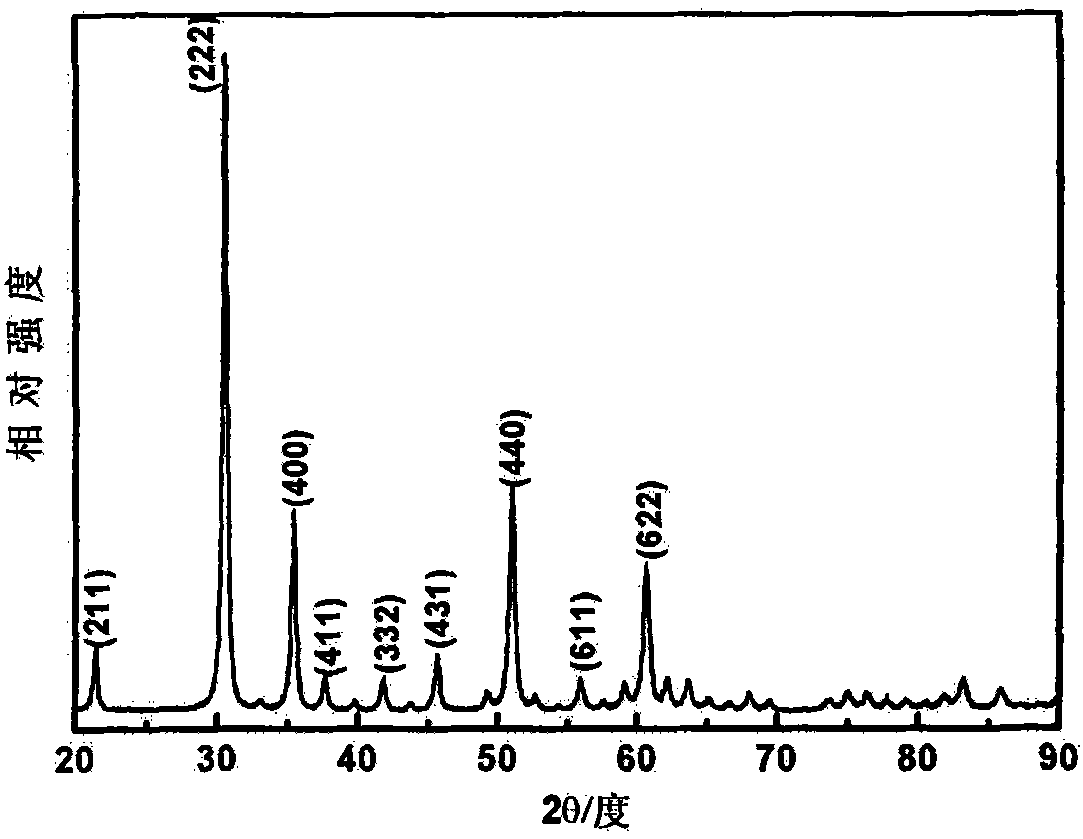
[0039] Weigh InCl 3 ·4H 2 O 0.5865g, urea 0.2401g(InCl 3 ·4H 2 The molar ratio of O to urea is 1:2), dissolved in 56ml of deionized water, magnetically stirred for 1 hour to form a colorless and transparent solution, transferred to a hydrothermal kettle, and reacted at 130°C for 18 hours. After the hydrothermal kettle is cooled to room temperature, take out the hydrothermal product. The hydrothermal product was separated by centrifugation, washed with deionized water and anhydrous ethanol three times, and then dried in an oven at 60°C for 2 hours to prepare a white precursor powder. figure 1 Based on the X-ray diffraction pattern of the precursor prepared in this example, it can be known by comparing with the standard card that the precursor obtained is cubic phase indium hydroxide (JCPDS No. 76-1463). figure 2 In the field emission scanning electron micrograph of the precursor indium hydroxide obtained in this example, it can be observed that the morphology of indium hydroxide...
Embodiment 2
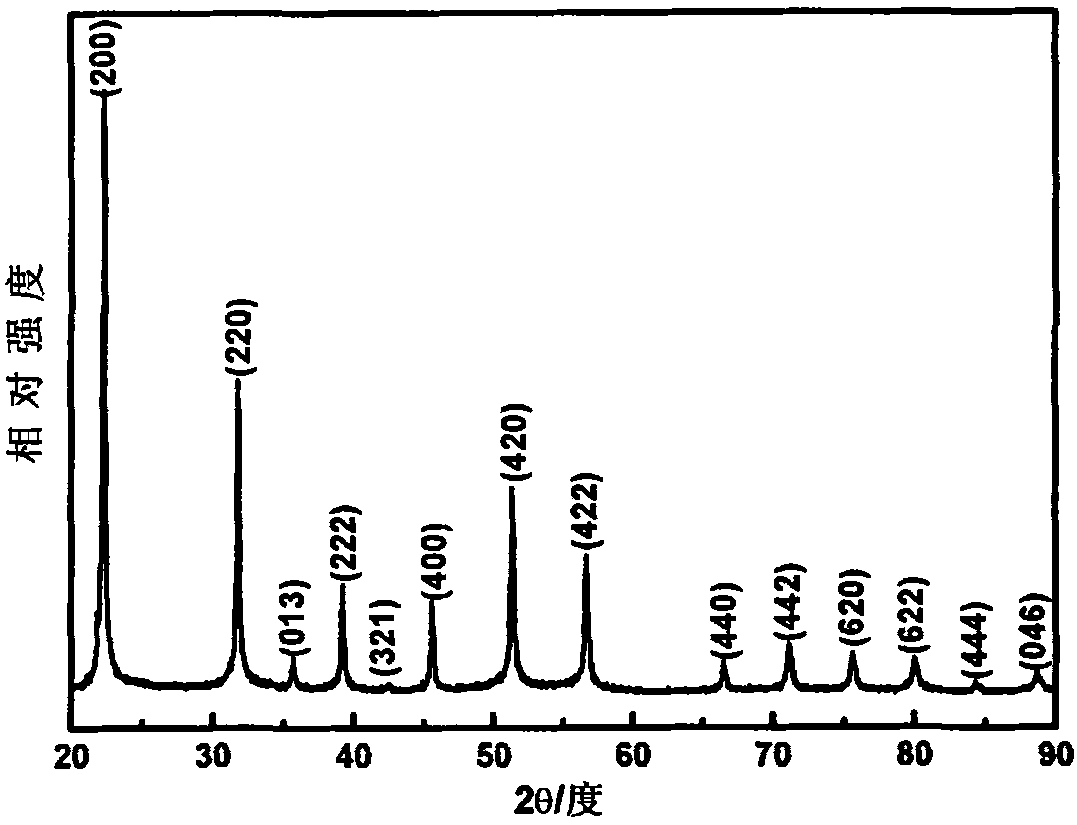
[0041] Weigh InCl 3 ·4H 2 O 0.5823g, urea 0.6006g (InCl 3 ·4H 2 The molar ratio of O to urea is 1:5), dissolved in 60ml of deionized water, magnetically stirred for 1 hour to form a colorless and transparent solution, transferred to a hydrothermal kettle, and reacted at 140°C for 12 hours. After the hydrothermal kettle is cooled to room temperature, take out the hydrothermal product. The hydrothermal product was separated by centrifugation, washed with deionized water and anhydrous ethanol successively three times, and then dried in an oven at 60°C for 3 hours to prepare a white precursor powder. The dried precursor indium hydroxide is calcined at 500° C. for 2 hours to prepare light yellow indium oxide powder. Figure 5 This is the X-ray diffraction pattern of the indium oxide powder obtained in this example. It can be seen from the comparison with the standard card that the prepared indium oxide is also cubic phase (JCPDS No. 06-0416). Image 6 The field emission scanning elec...
Embodiment 3
[0043] Weigh InCl 3 ·4H 2 O 0.5834g, urea 0.9610g (InCl 3 ·4H 2 The molar ratio of O to urea is 1:8), dissolved in 58ml of deionized water, magnetically stirred for 1 hour to form a colorless and transparent solution, transferred to a hydrothermal kettle, and kept at 120°C for 18 hours to react. After the hydrothermal kettle is cooled to room temperature, take out the hydrothermal product. The hydrothermal product was separated by centrifugation, washed with deionized water and anhydrous ethanol for 3 times, and then dried in an oven at 60°C for 4 hours to prepare a white precursor powder. Figure 7 Is the field emission scanning electron microscope photo of the precursor indium hydroxide prepared in this example, and figure 2 , 6 In comparison, it is not difficult to find that the square-shaped indium hydroxide disappears and is replaced by flower-like clusters or single microrods composed of micro / nanorods with diameters between 300nm and 2μm. The dried precursor indium hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com