Method for detecting impurities in freeze-dried powder injection of nedaplatin
A technology of freeze-dried powder injection and detection method, which is applied in the field of impurity detection of nedaplatin freeze-dried powder injection, can solve problems such as no improvement, and achieve the effects of avoiding hidden dangers, prolonging retention time, and improving durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Instrument: SHIMADZULC-20AT high performance liquid chromatography;
[0041] Chromatographic column: octylsilane bonded silica gel column (4.6×250mm, 5μm);
[0042] Mobile phase: prepare a mixture with water-acetonitrile (volume ratio 80:20), add 0.1% diethylamine in the total volume of the mixture to the mixture;
[0043] Column temperature: 45°C;
[0044] Flow rate: 0.5ml / min;
[0045] Detection wavelength: 220nm.
[0046] Preparation of sample solution: The nedaplatin freeze-dried powder injection sample was prepared to contain nedaplatin 1mg / ml by using a mixed solvent of water and methanol (volume ratio of 50:50, and adjusting the pH to 5.6 with 0.05mol / L phosphoric acid). solution, filtered and stored at 8°C in the dark;
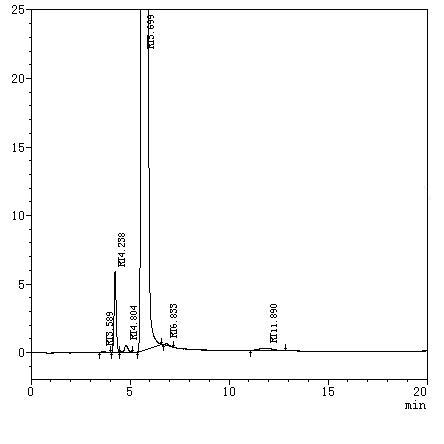
[0047] Determination: Inject 5 μL of sample solution into a high-performance liquid chromatograph, record and analyze the chromatogram, see figure 1 . The test results showed that the impurities were well separated.
Embodiment 2
[0049] Instrument: SHIMADZULC-20AT high performance liquid chromatography;
[0050] Chromatographic column: octylsilane bonded silica gel column (4.6×250mm, 5μm);
[0051] Mobile phase: prepare a mixture with water-acetonitrile (volume ratio 90:10), add 0.005% triethylamine of the total volume of the mixture to the mixture;
[0052] Column temperature: 40°C;
[0053] Flow rate: 1.0ml / min;
[0054] Detection wavelength: 210nm.
[0055] Preparation of sample solution: Nedaplatin freeze-dried powder injection samples were prepared to contain nedaplatin 0.5mg / ml by using a mixed solvent of water and methanol (volume ratio of 50:50, and adjusting the pH to 5.6 with 0.05mol / L phosphoric acid) solution, filtered and stored at 8°C in the dark;
[0056] Determination: Inject 20 μL of the sample solution into a high-performance liquid chromatograph, record and analyze the chromatogram, and the test results show that the impurities are well separated.
Embodiment 3
[0058] Instrument: SHIMADZULC-20AT high performance liquid chromatography;
[0059] Chromatographic column: octylsilane bonded silica gel column (4.6×250mm, 5μm);
[0060] Mobile phase: prepare a mixture with water-methanol (volume ratio 70:30), add 0.05% diethylamine in the total volume of the mixture to the mixture;
[0061] Column temperature: 30°C;
[0062] Flow rate: 0.8ml / min;
[0063] Detection wavelength: 205nm.
[0064] Preparation of sample solution: The nedaplatin freeze-dried powder injection sample was prepared to contain nedaplatin 2mg / ml by using a mixed solvent of water and methanol (volume ratio of 50:50, and adjusting the pH to 5.6 with 0.05mol / L phosphoric acid). solution, filtered and stored at 2°C in the dark;
[0065] Determination: Inject 5 μL of the sample solution into a high-performance liquid chromatograph, record the chromatogram and analyze it. The test results show that the impurities are well separated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com