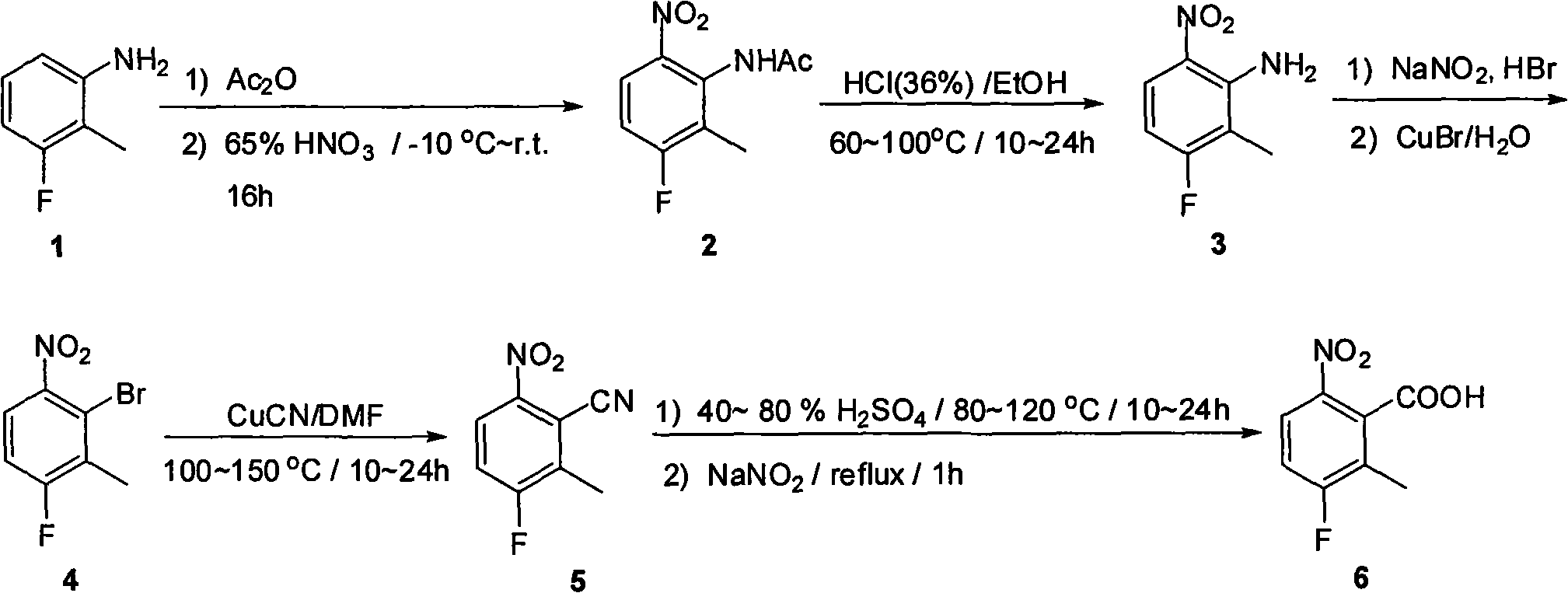
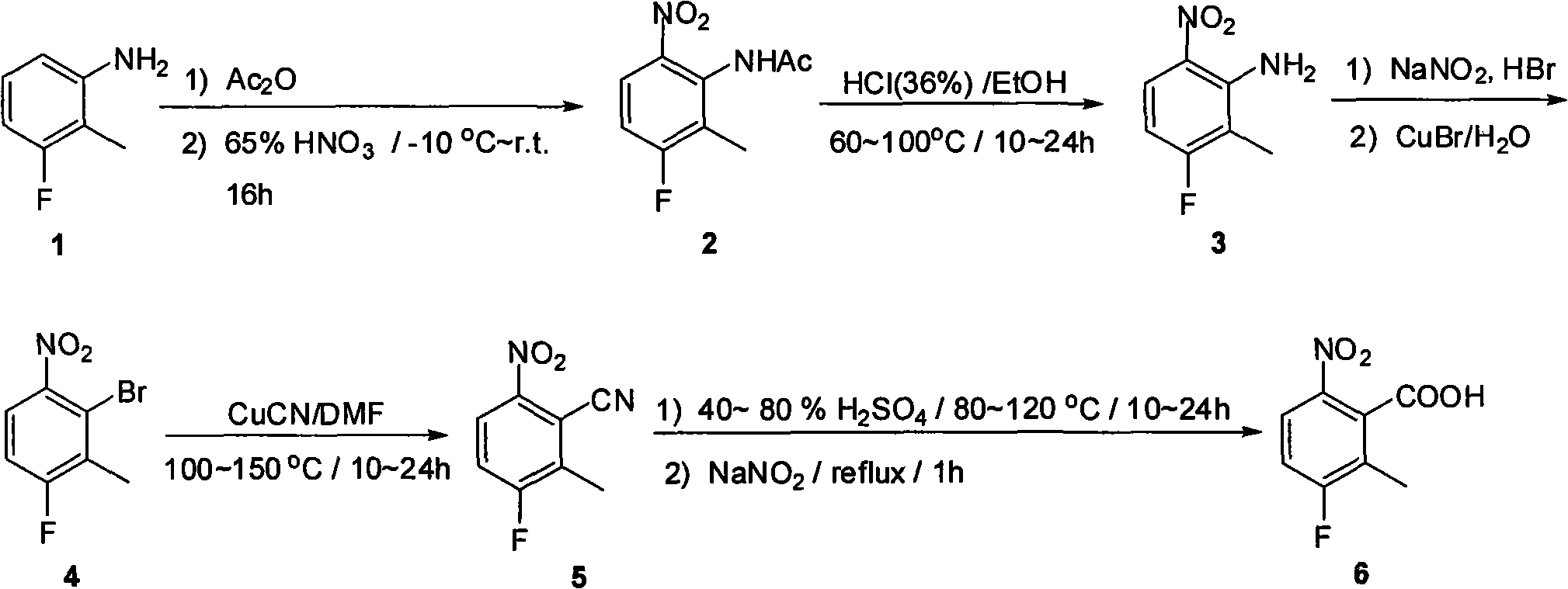
Synthesis method of 2 - methyl -3 - fluoride - 6 -nitrobenzoic acid
A technology for the synthesis of nitrobenzoic acid and its synthesis method, which is applied in the field of synthesis of important pharmaceutical intermediate 2-methyl-3-fluoro-6-nitrobenzoic acid, and can solve the problems of high synthesis cost, complex post-processing and lack of synthesis methods and other problems, to achieve the effect of fast and convenient raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
[0015] 1. Synthesis of N-(2-methyl-3-fluoro-6-nitrophenyl)acetamide 2
[0016] 2-Methyl-3-fluoroaniline 1 (600g, 4.8mol) was dissolved in acetic anhydride (3000mL), then the solution was cooled to -10°C, at this temperature 65% by weight of concentrated nitric acid (375mL) was slowly Add dropwise to the reaction solution. After 3 hours, the dropwise addition was completed, and the reaction solution naturally rose to room temperature, and then stirred for 16 hours. The solid precipitated in the reaction solution was filtered to obtain 250 g of the product. The mother liquor was concentrated to dryness again to obtain a crude product, which was recrystallized from ethanol to obtain another 550 g of pure N-(2-methyl-3-fluoro-6-nitrophenyl)acetamide 2. (Yield: 78%).
[0017] H NMR spectrum 1 H-NMR (DMSO, 400MHz), δppm: 9.99 (s, 1H), 7.82 (dd, J 1 =8.8Hz,J 2 =5.6Hz, 1H), 6.50(t, J=8.8Hz, 1H), 2.12(s, 3H), 2.02(s, 3H).
[0018] 2. Synthesis of 2-methyl-3-fluoro-6-...
Embodiment 2
[0031] The second hydrolysis reaction is to dissolve N-(2-methyl-3-fluorophenyl)acetamide 2 in ethanol and concentrated hydrochloric acid, and heat for 15 hours to obtain 2-methyl-3-fluoro-6-nitroaniline 3.
[0032] The third step of the diazotization reaction is that 2-methyl-3-fluoro-6-nitroaniline 3 is dissolved in a 48% hydrobromic acid solution by weight, and an aqueous solution of sodium nitrite is added dropwise at 0°C. React at 0°C for 30 minutes, and add dropwise to the mixed solution of cuprous bromide and water. Reaction at 80°C for 2 hours gave 2-bromo-3-methyl-4-fluoro-nitrobenzene 4.
[0033] The fourth step reaction is to dissolve 2-bromo-3-methyl-4-fluoro-nitrobenzene 4 in N,N-dimethylformamide, add cuprous cyanide, and react at 130°C for 17 hours to obtain 2-Methyl-3-fluoro-6-nitrobenzonitrile 5.
[0034] The fifth step reaction is to dissolve 2-methyl-3-fluoro-6-nitrobenzonitrile 5 in 80% sulfuric acid by weight and react at 90°C for 18 hours, then react f...
Embodiment 3
[0037]The second step of hydrolysis reaction is to dissolve N-(2-methyl-3-fluorophenyl)acetamide 2 in ethanol and concentrated hydrochloric acid, and heat for 18 hours to obtain 2-methyl-3-fluoro-6-nitroaniline 3.
[0038] The third step of the diazotization reaction is that 2-methyl-3-fluoro-6-nitroaniline 3 is dissolved in a 48% hydrobromic acid solution by weight, and an aqueous solution of sodium nitrite is added dropwise at 0°C. React at 0°C for 30 minutes, and add dropwise to the mixed solution of cuprous bromide and water. Reaction at 100°C for 1 hour afforded 2-bromo-3-methyl-4-fluoro-nitrobenzene 4.
[0039] The fourth step reaction is to dissolve 2-bromo-3-methyl-4-fluoro-nitrobenzene 4 in N,N-dimethylformamide, add cuprous cyanide, and react at 110°C for 20 hours to obtain 2-Methyl-3-fluoro-6-nitrobenzonitrile 5.
[0040] The fifth step reaction is to dissolve 2-methyl-3-fluoro-6-nitrobenzonitrile 5 in 60% sulfuric acid by weight and react at 110°C for 12 hours, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com