Use of human antibody capable of neutralizing hepatitis b virus for the prevention or treatment of hepatitis b virus infection
A hepatitis B virus and human antibody technology, applied in the direction of antibodies, antiviral agents, medical preparations containing active ingredients, etc., can solve problems such as low specificity, non-therapeutic antibody source, infectious pathogen contamination, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
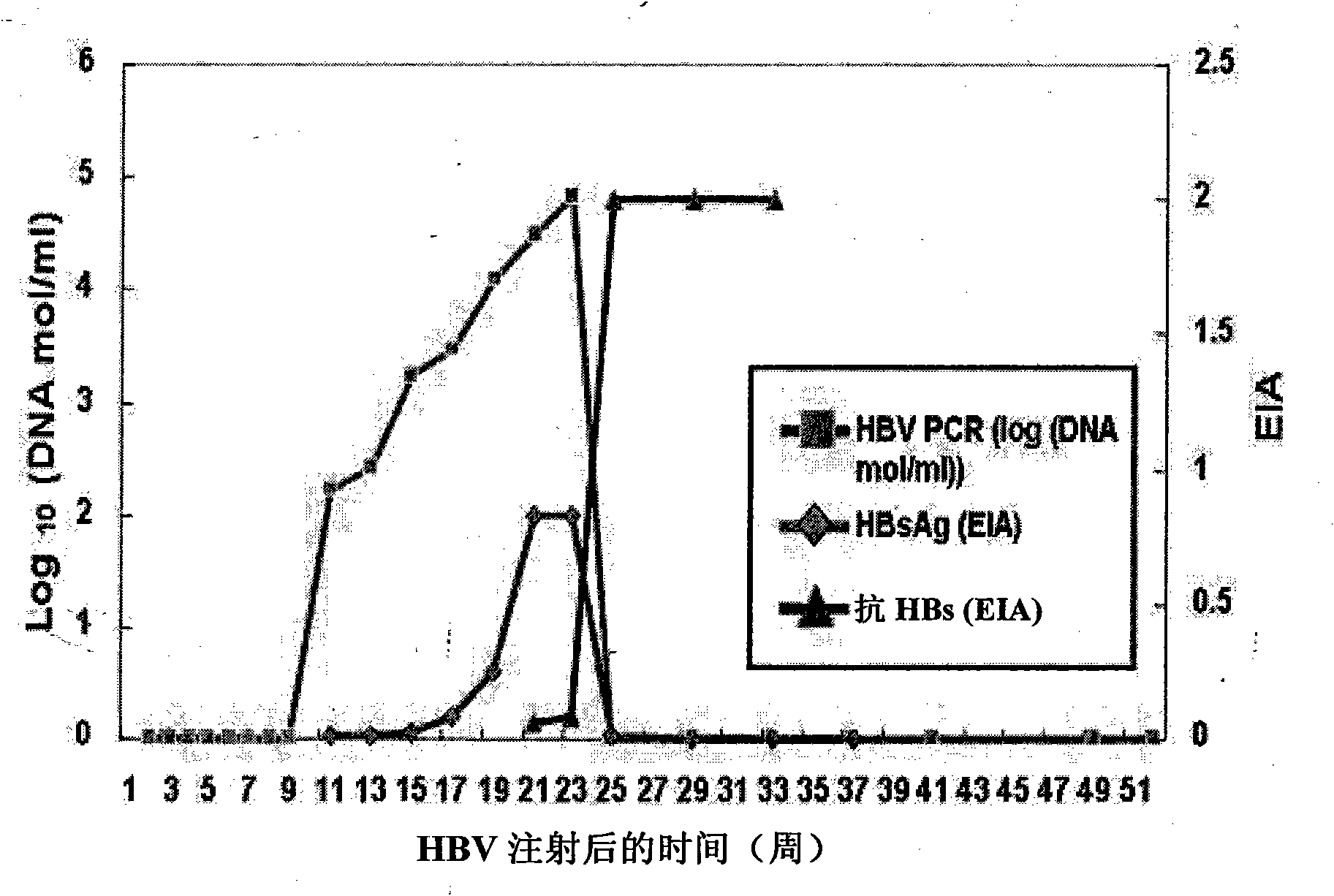
[0030] Example 1: Evaluation of the ability to neutralize HBV in chimpanzees
[0031] In order to confirm that the human antibodies of the present invention exhibit the ability to neutralize HBV in vivo, the following experiments were performed.
[0032] HBV 100 CID50 (50% chimpanzee infectious dose) obtained from Hepatitis Research Foundation (New York, USA) was put into three tubes. 0.1 mg and 10 mg of the antibody of the present invention (Hepabig-Gene, Green Cross, Korea) were added to two tubes, respectively, and no antibody was added to the other tube. PBS (Phosphate Buffered Saline) was added to the tube to a final volume of 3 ml, the mixture was incubated at 37°C for 1 hour, then at 4°C overnight, and frozen with liquid nitrogen to prepare test samples.
[0033] The test samples were administered intravenously to three chimpanzees (Hepatitis Research Foundation, New York, USA) who had not been previously infected with HBV. Antibody doses for each chimpanzee are lis...
Embodiment 2
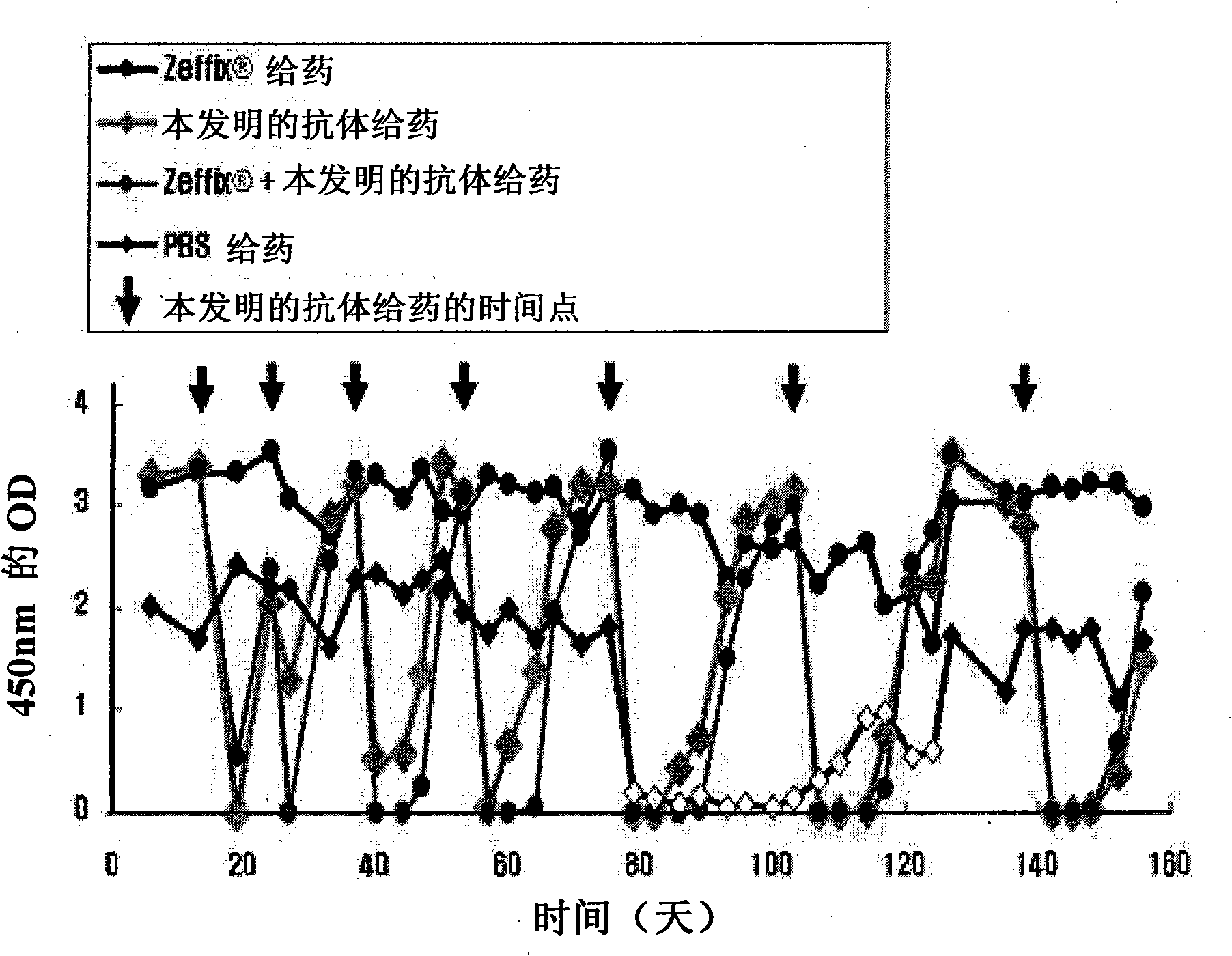
[0048] Example 2: Evaluation of HBV Neutralization Ability in Mouse Model
[0049] 2-1) Construction of plasmid containing HBV DNA
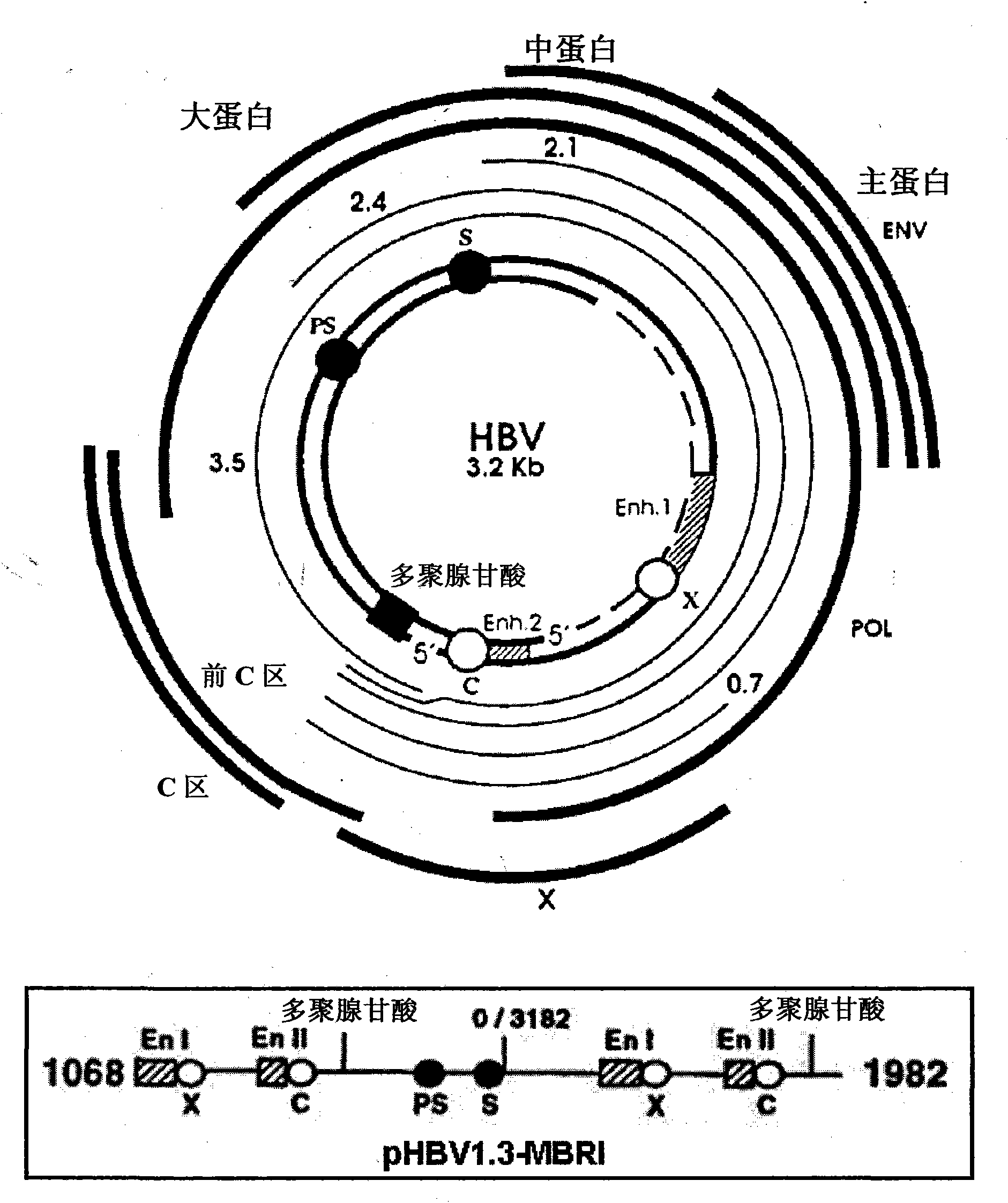
[0050] The 1.3 sequence (Gene Bank accession No.DQ683578) of the HBV (adr subtype) gene (the HBV gene from the upstream of the enhancer I of the HBV genome to the downstream of the polyadenylation region; see figure 2 ) was inserted into the PmeI restriction site of pcDNA3.1 (Invitrogen, USA), and the resulting plasmid pHBV1.3-MBRI was prepared by using EndoFree PlasmidKit (Qiagen, Germany).
[0051] 2-2) Plasmid injection
[0052] 20 μg of the pHBV1.3-MBRI plasmid prepared in 2-1) was dissolved in physiological saline solution to a volume corresponding to 9% of the mouse weight, and injected into immunodeficiency at a rate of 0.3 ml / sec (hydrodynamic injection). In the tail vein of C57BL / 6J-Prkdcscid / SzJ female mice (8 weeks old, Jacksonlaboratory, USA).
[0053] 2-3) Administration of the antibody of the present invention and HBV replicat...
Embodiment 3
[0061] Example 3: Immunoprecipitation analysis of antibodies for HBV binding ability
[0062] Immunoprecipitation was performed to examine whether the antibody of the present invention binds to HBV in the blood of hepatitis B patients (provided by Ajou University, School of Medicine, Korea).
[0063] 3-1) Preparation of blood samples from patients with hepatitis B
[0064] 1000 μl of blood samples from patients with hepatitis B (diluted 10 times with 0.2% BSA / PBS buffer solution) were incubated with goat anti-human IgG (Fc-specific) agarose conjugate (Research Diagnostics Inc., Flanders, NJ) to remove immunoglobulins .
[0065] 3-2) Binding reaction between the antibody of the present invention and goat anti-human IgG agarose conjugate
[0066] 10 μl of antibodies of the invention (0.1, 0.5, 1 and 5 μg) and PBS were mixed with 50 μl of goat anti-human IgG agarose conjugate (Research Diagnostics) and incubated for 1 hour at room temperature with stirring. 10 mg of human im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com