Lornoxicam hydrogel patch and preparation method thereof
A lornoxicam hydrogel patch and technology of lornoxicam, applied in anti-inflammatory agents, pharmaceutical formulations, non-central analgesics, etc., can solve the problems of slow drug release and inability to fully meet clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
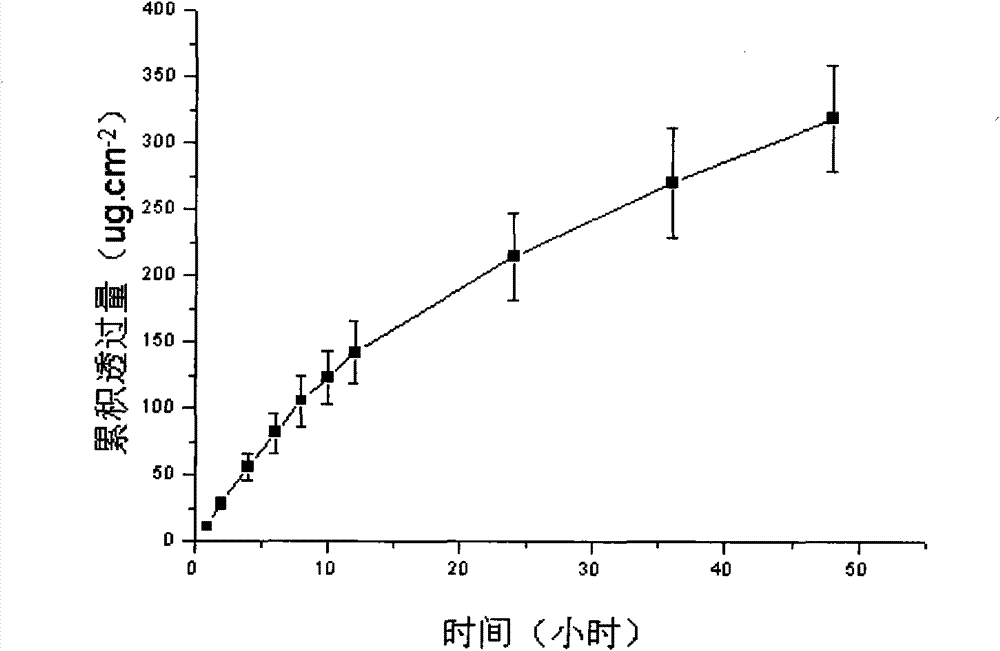
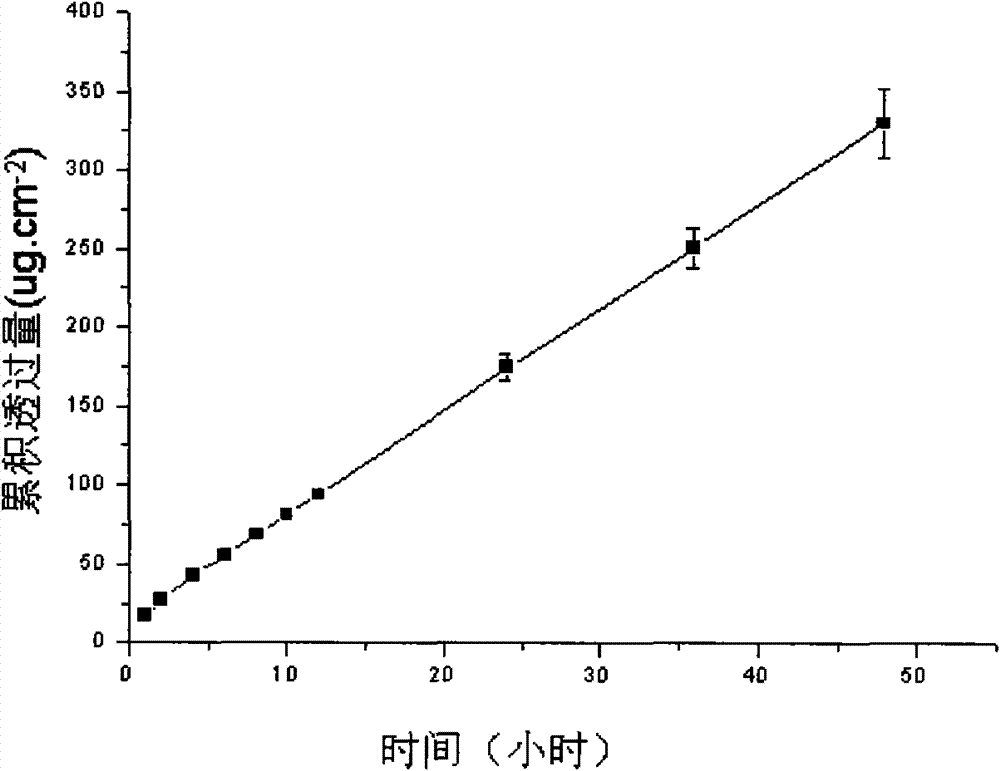
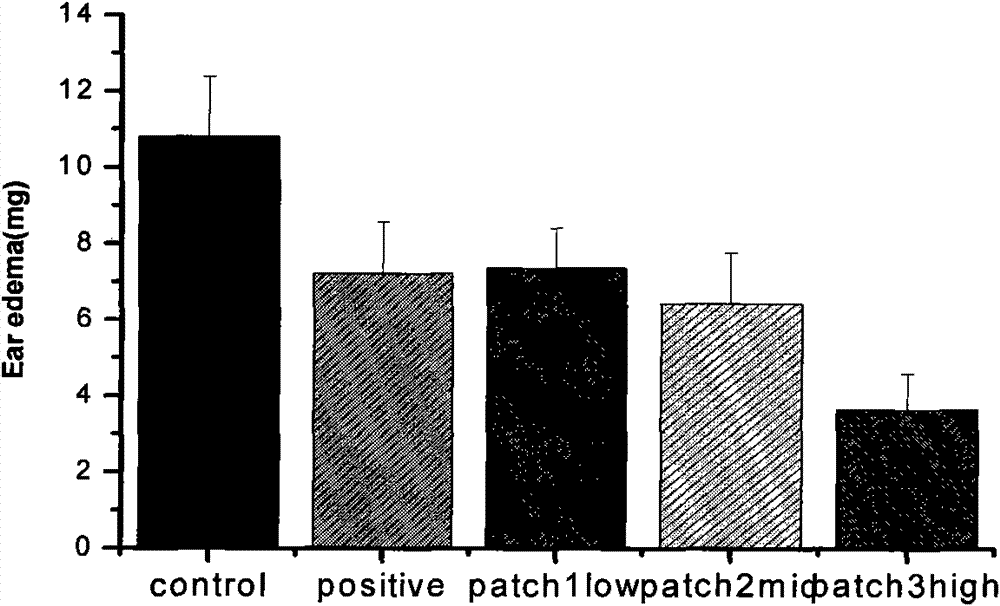
Image
Examples
Embodiment 1
[0039] Example 1, preparation of lornoxicam hydrogel patch
[0040] Drug-containing matrix components (per 100cm 2 )
[0041] Lornoxicam (Main Drug) 0.05g
[0042] Polyvinylpyrrolidone K90 (skeleton material) 1.05g
[0043] Polyvinylpyrrolidone K30 (skeleton material) 0.78g
[0044] Polyvinyl alcohol-124 (skeleton material) 0.26g
[0045] Aluminum trichloride (crosslinking agent) 0.015g
[0046] Glycerin (humectant) 1.57g
[0047] Azone (penetration enhancer) 1g
[0048] Propylene glycol (penetration enhancer) 1g
[0049] Deionized water 8.35g
[0050] Preparation:
[0051](1) Drug-containing base: add deionized water to the recipe quantities of polyvinylpyrrolidone K90, polyvinylpyrrolidone K30, polyvinylalcohol-124, aluminum trichloride, and glycerin, and heat and stir at 90°C to mix evenly for about 2 hours. Gradually cool down until it forms a gel at room temperature to obtain phase I; dissolve lornoxicam completely with an appropriate amount of DMF, then add azo...
Embodiment 2
[0052] Example 2, preparation of lornoxicam hydrogel patch
[0053] Drug-containing matrix components (per 100cm 2 )
[0054] Lornoxicam (Main Drug) 0.1g
[0055] Polyvinylpyrrolidone K90 (skeleton material) 1.05g
[0056] Polyvinylpyrrolidone K30 (skeleton material) 0.78g
[0057] Polyvinyl alcohol-124 (skeleton material) 0.26g
[0058] Aluminum trichloride (crosslinking agent) 0.015g
[0059] Glycerin (humectant) 1.57g
[0060] Azone (penetration enhancer) 1g
[0061] Propylene glycol (penetration enhancer) 1g
[0062] Deionized water 8.35g
[0063] The preparation method is the same as in Example 1.
Embodiment 3
[0064] Example 3, preparation of lornoxicam hydrogel patch
[0065] Drug-containing matrix components (per 100cm 2 )
[0066] Lornoxicam (Main Drug) 0.05g
[0067] Polyvinylpyrrolidone K90 (skeleton material) 1.31g
[0068] Polyvinylpyrrolidone K30 (skeleton material) 0.98g
[0069] Polyvinyl alcohol-124 (skeleton material) 0.325g
[0070] Aluminum trichloride (crosslinking agent) 0.018g
[0071] Macrogol 400 (humectant) 2.12g
[0072] Azone (penetration enhancer) 1g
[0073] Propylene glycol (penetration enhancer) 1g
[0074] Deionized water 10.44g
[0075] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com