Split heterocyclic 23-hydroxybetulinic acid derivatives and preparation method, preparation and application thereof
A kind of derivative, betulinic acid technology, applied in the field of natural medicine and medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
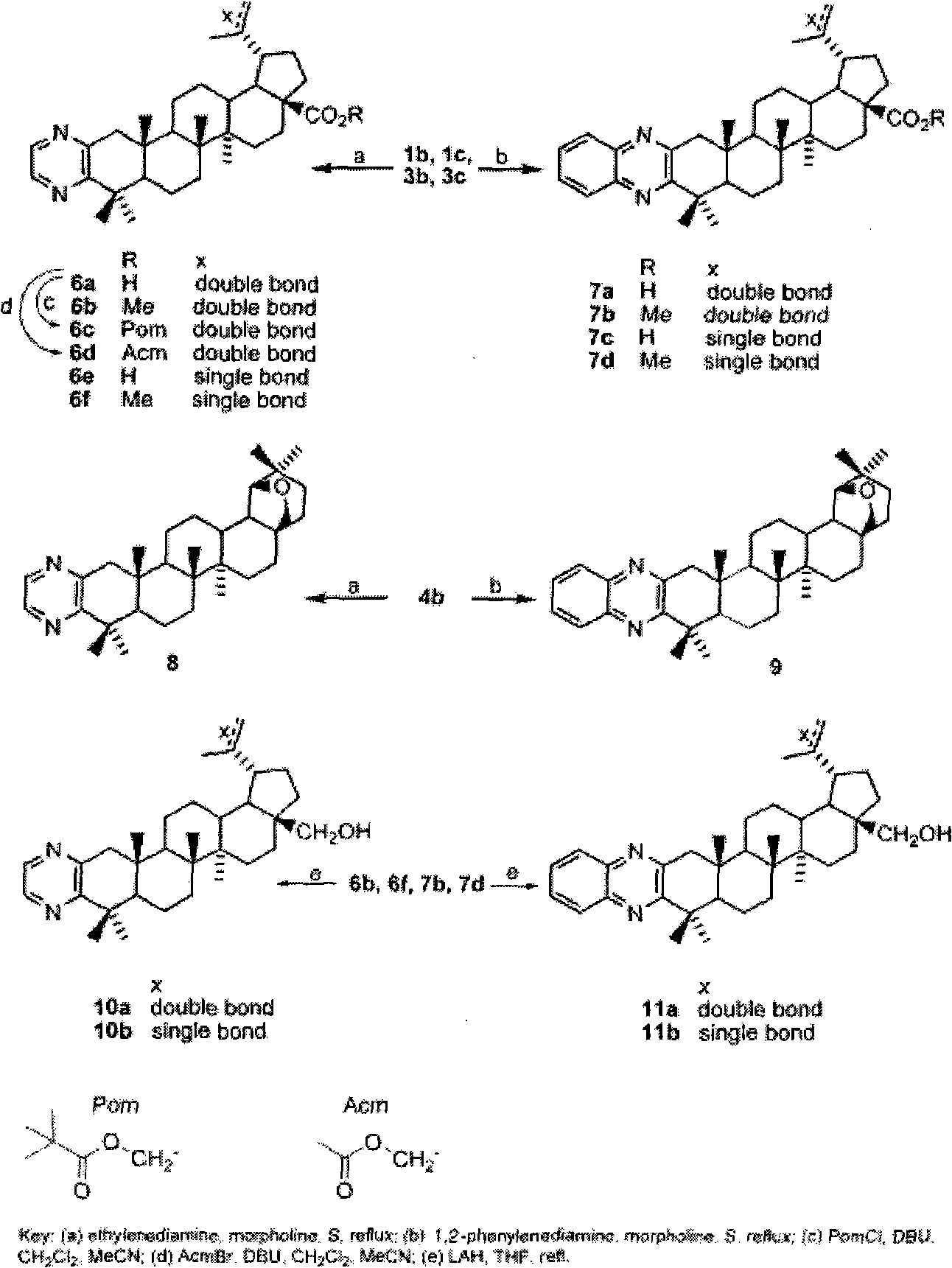
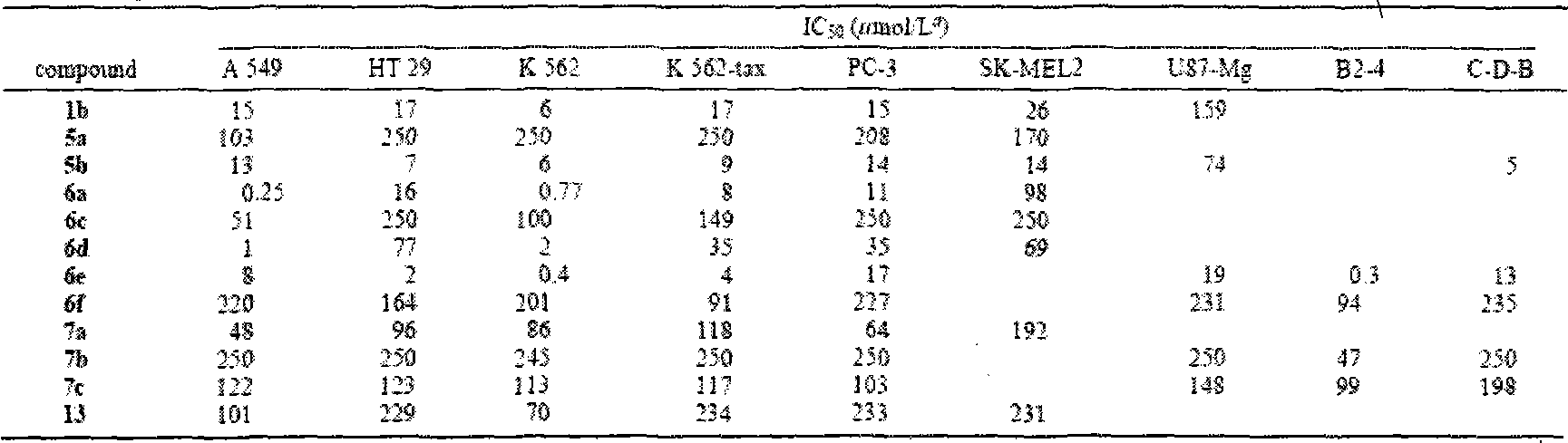
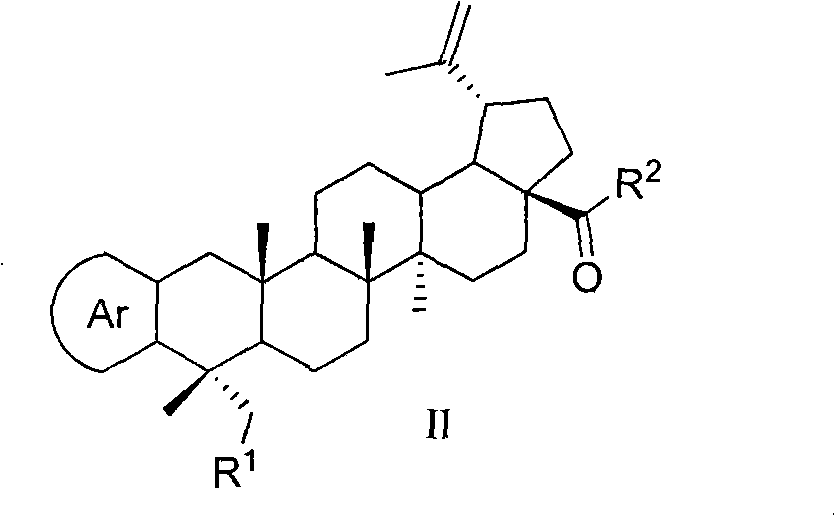
[0081] 23-tert-butyldimethylsilyloxy-lupine-20(29)-en-28-oic acid benzyl a[3,2-b]pyrazine
[0082] Dissolve 3-carbonyl-23-tert-butyldimethylsilyloxy-lupine-20(29)-en-28-oic acid benzyl ester (0.6g, 0.89mmol) in morpholine (25ml) and add sulfur (0.32g, 10mmol) and ethylenediamine (0.28g, 4.5mmol), refluxed for 4h, diluted with ethyl acetate (30ml), washed three times with water, washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, column Chromatography (petroleum ether: ethyl acetate = 20:1) gave a white solid (0.43 g, 68%). ESI-MS m / z: 711.5[M+H] +
Embodiment 2
[0084] 23-Hydroxy-lupine-20(29)-en-28-oic acid benzyl[3,2-b]pyrazine
[0085] Dissolve 23-tert-butyldimethylsilyloxy-lupin-20(29)-ene-28-oate benzyl[3,2-b]pyrazine (0.4g, 0.56mmol) in acetone (30ml ), add 10% hydrochloric acid (2ml), stir at room temperature for 2 hours, dilute with ethyl acetate, wash with water until neutral, wash with saturated brine, dry over anhydrous sodium sulfate, filter, concentrate, column chromatography (petroleum ether: ethyl acetate =2:1), a white solid (0.30g, 91%) was obtained, mp 118-121°C. IR (film, cm -1 )3438, 3069, 2951, 2871, 1727, 1644, 1456, 1407, 1379, 1171, 1156, 1130, 885, 699; 1 H-NMR (CDCl 3 , 300MHz): δ0.84, 0.99, 1.30, 1.70 (6H for 0.84, each 3H for others, s, 24, 25, 26, 27 and 30-CH 3 ), 2.22-2.31(2H, m), 2.44(1H, d, J=16.9Hz, H-1a), 3.03(1H, m, H-19), 3.08(1H, d, J=16.7Hz, H -1b), 3.47, 3.79 (each 1H, d, J=10.5Hz, H-23), 4.63, 4.75 (each 1H, s, H-29), 5.13 (2H, dd, J=22.7, 12.3Hz, CH 2 -Ar), 7.31-7.38 (5H, m, H-Ar), 8.35,...
Embodiment 3
[0087] 23-Acetoxy-lupine-20(29)-en-28-oic acid benzyl[3,2-b]pyrazine
[0088] 23-Hydroxy-lupine-20(29)-ene-28-acid benzyl[3,2-b]pyrazine (0.25g, 0.43mmol) was dissolved in pyridine (10ml), and acetic anhydride (2ml ), stirred at room temperature for 10h, diluted with ethyl acetate (25ml), washed with 10% hydrochloric acid until acidic, washed twice with water, washed with saturated sodium bicarbonate until no bubbles were produced, dried over anhydrous sodium sulfate, filtered, concentrated, column chromatography (petroleum ether:ethyl acetate=6:1) to obtain a white solid (0.23g, 86%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com