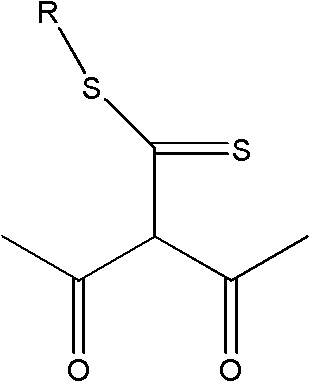
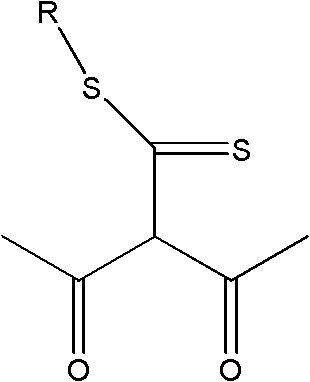
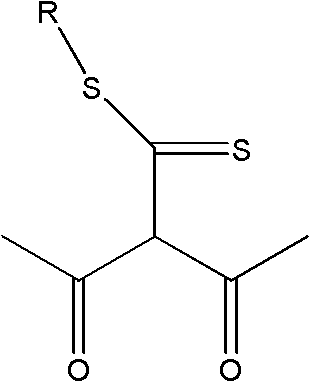
Application of extractant and method for extracting zinc from ammonia solution
An extraction agent and extraction technology, applied in the field of metallurgy, to achieve the effect of fast extraction speed and fast phase separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) The total concentration of ammonia and ammonium in the ammoniacal zinc-containing aqueous solution is 2.00mol L -1 , ammonia concentration 0.11mol L -1 , pH=8; its zinc content is: ①Zn-NH 3 -NH 4 HCO 3 -H 2 The concentration of zinc in O solution is 6.03g L -1 ; ②Zn-NH 3 -NH 4 Cl-H 2 The concentration of zinc in O solution is 6.18g L -1 ;③Zn-NH 3 -(NH 4 ) 2 SO 4 -H 2 The concentration of zinc in O solution is 5.95g L -1 .
[0020] (2) Extractant composition: 40% (volume ratio) 2-acetyl-3-oxo-dithiobutanoic acid-dodecyl ester, mixed with 55% sulfonated kerosene and 5% TBP to form an organic phase
[0021] (3) Extraction and stripping operating conditions: at normal temperature, the ammoniacal zinc-containing aqueous solution in (1) and the extractant in (2) are respectively carried out primary extraction by comparison A / O=1: 1, and the extraction time for 5 minutes; then use sulfuric acid to back-extract the zinc-containing extracted organic phase, an...
Embodiment 2
[0024] Zn-NH 3 -(NH 4 ) 2 SO 4 -H 2 O solution, in which the concentration of zinc is 11.20g L -1 , the total concentration of ammonia and ammonium is 3mol L -1 , ammonia concentration 1.00mol L -1 , pH=11. The organic phase was composed of 50% (by volume) 2-acetyl-3-oxo-dithiobutanoic acid-hexadecyl ester and 50% sulfonated kerosene. At normal temperature, carry out the primary extraction with the water phase according to the ratio A / O=2:1, and the extraction time is 5min; carry out the third stage according to the ratio A / O=1:1 for the sulfuric acid back-extraction of the extracted organic phase containing zinc Back extraction, the back extraction time is 3min. The primary extraction rate of zinc is ≥95%, the highest is 98.2%, the extraction distribution ratio is D≥19, the zinc primary stripping is only 58.1%, and after the tertiary stripping, it can be as high as 97%. The extraction and stripping phase separation is fast, the phase interface is clear, and there is ...
Embodiment 3
[0026] Zn-NH 3 -(NH 4 ) 2 SO 4 -H 2 O solution, in which the concentration of zinc is 16.680g·L -1 , the total concentration of ammonia and ammonium is 2.13mol L -1 , ammonia concentration 0.24mol L -1, pH=9. Mix 60% (volume ratio) of 2-acetyl-3-oxo-dithiobutanoic acid-tetradecyl ester alkane with 36% sulfonated kerosene and 4% TBP to form the organic phase. At normal temperature, carry out primary extraction with the water phase according to the ratio A / O=2:1, and the extraction time is 5min; The stripping time is 3min. The primary extraction rate of zinc is ≥ 97%, the highest is 99.12%, the extraction distribution ratio D is ≥ 32.3, the highest is 112.6; after the third back extraction, it can be as high as more than 97%; the extraction and back extraction phase separation is fast, the phase interface is clear, no Emulsification or third phase generation. After the three-stage circular extraction of zinc, the concentration of zinc in the back extraction water phase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com