Solid super acidic catalyst and preparation method thereof
A solid superacid and catalyst technology, which is applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of difficult catalyst recovery, low catalytic efficiency, easy deactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
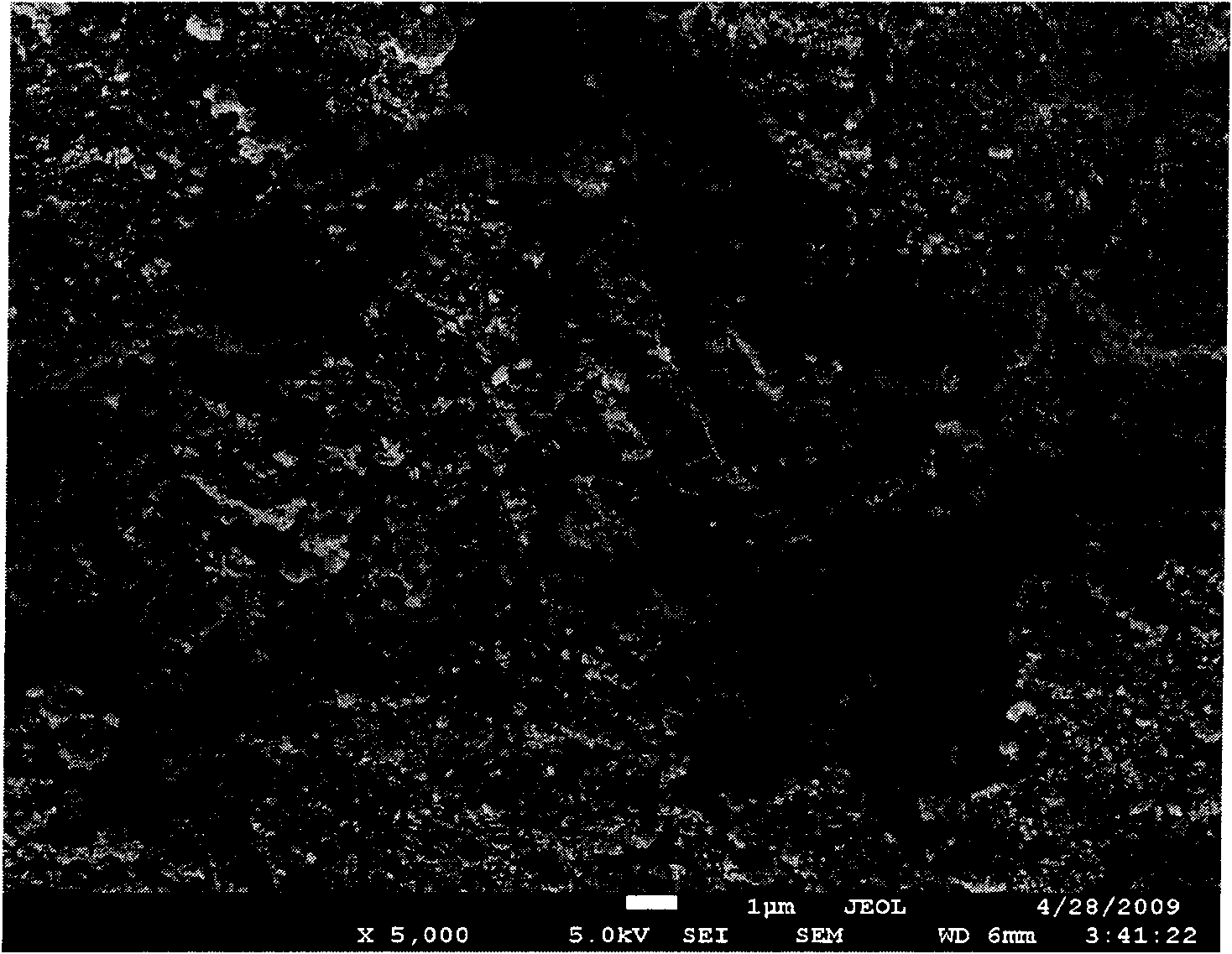
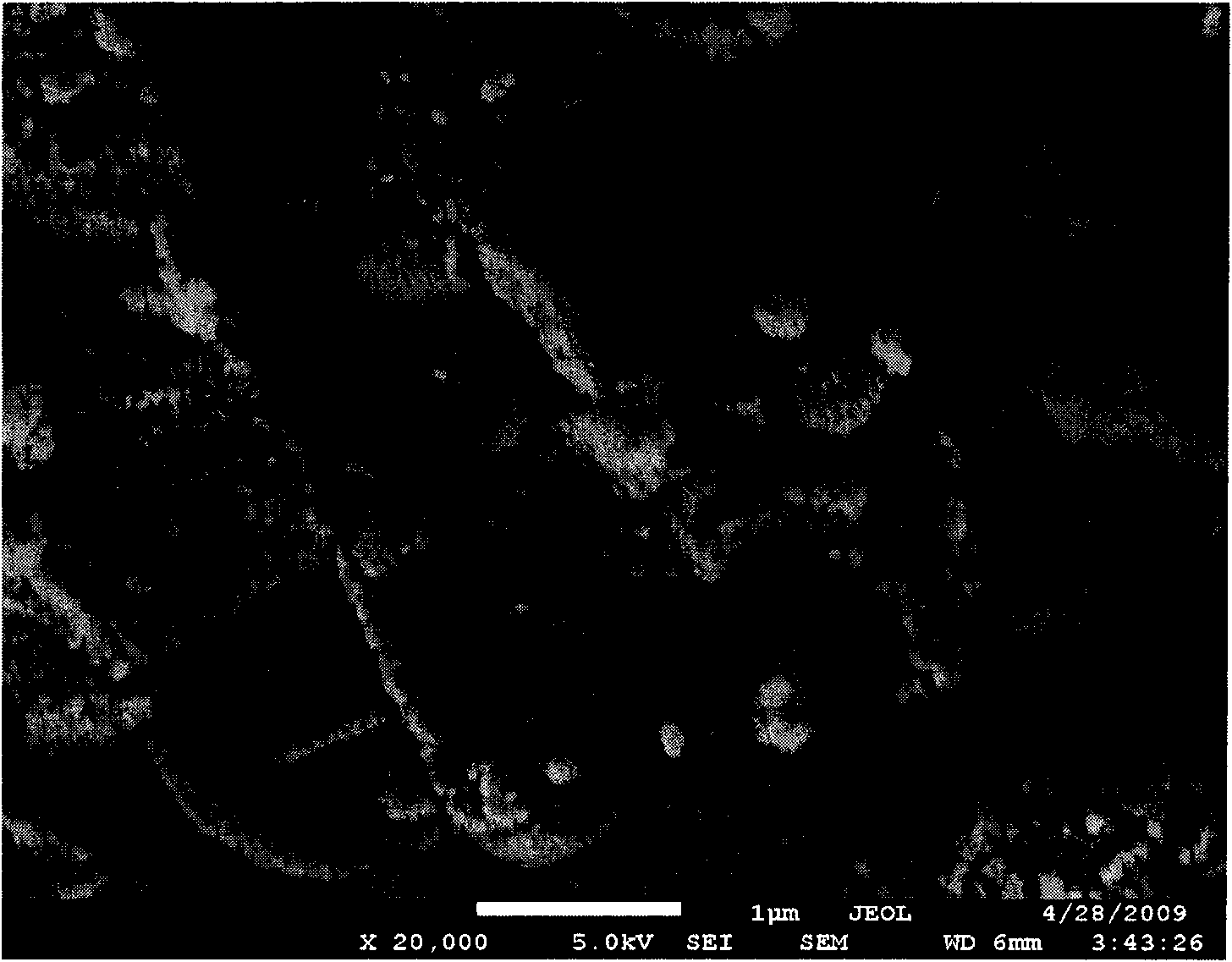
Image
Examples
Embodiment 1
[0065] Mix 52g of second-line oil and 12g of SP-80, heat to 80°C and mix evenly; heat 375g of aluminum nitrate nonahydrate and 320g of urea to 80°C to melt, drop into the above mixture, and mix for 15 minutes to form a uniform supersolubilized colloid. Seal the reactor and react at 120° C. for 4 hours to obtain nano-aluminum hydroxide gel. Heat 31g of common three-line oil, 7g of minus three-line wax paste, and 6g of polyisobutenyl diethylene glycol maleate to 80°C and mix evenly. 110g of zirconium oxychloride octahydrate is dropped into the above mixture, mix evenly, and keep at 80°C. Then 130 g of ammonia water with a concentration of 40 wt % was added dropwise to obtain a nano zirconium hydroxide gel, which was washed and centrifuged. The nano zirconium hydroxide gel was impregnated with H at a concentration of 0.5 mol / L 2 SO 4 Solution for 0.5 hours, mix well, and dry at 110°C for 12 hours. Mix 20 g of nano aluminum hydroxide dry powder, 60 g of nano zirconium hydroxide...
Embodiment 2
[0067] Mix 140g minus three line oil and 12g SP-60, heat to 100°C and mix well; heat 375g aluminum nitrate nonahydrate and 320g urea to 80°C to melt, drop into the above mixture, and mix for 15 minutes to form a uniform supersolubilized colloid. Seal the reactor and react at 120° C. for 4 hours to obtain nano-aluminum hydroxide gel. Heat 31g of minus three line oil, 23g of minus three line wax paste, 14g of polyisobutenyl diethylene glycol maleate to 80°C and mix evenly, heat and dissolve 240g of zirconium oxychloride octahydrate and 280g of urea, drop them into the above mixture, mix Evenly, keep at 100°C, then dropwise add 130g of ammonia water with a concentration of 40wt%, to obtain nano zirconium hydroxide gel, wash and centrifuge, and dry at 110°C for 8h. Dry the nano-zirconium hydroxide gel and impregnate the H at a concentration of 1.5mol / L 2 SO 4 The solution was mixed for 5 hours, and dried at 110°C for 12 hours. Mix 30g of nano-aluminum hydroxide gel dry powder, ...
Embodiment 3
[0069] Mix 83g of second-line oil and 34g of polyisobutenyl triethanolamine maleate, heat to 90°C and mix evenly; heat 750g of aluminum nitrate nonahydrate and 350g of urea to 90°C to melt, drop into the above mixture, and mix for 15 minutes to form a uniform Supersolubilize the colloid, seal the reactor, and react at 120°C for 4 hours to obtain nano-aluminum hydroxide gel. Heat 49g of common three-line oil, 21g of minus three-line wax paste, and 22g of polyisobutenyl triethylene glycol maleate to 80°C and mix evenly. Heat and drop 950g of zirconium oxychloride octahydrate into the above mixture, mix evenly, and keep at 80°C , and then add 410 g of liquid ammonia to obtain a nano zirconium hydroxide gel, which is washed and centrifuged. The nano zirconium hydroxide gel was impregnated with 1.0mol / L H 2 SO 4 The solution was mixed for 8 hours, and dried at 110°C for 12 hours. Mix 50 g of nanometer aluminum hydroxide gel dry powder, 40 g of nanometer zirconium hydroxide gel d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pore volume | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com