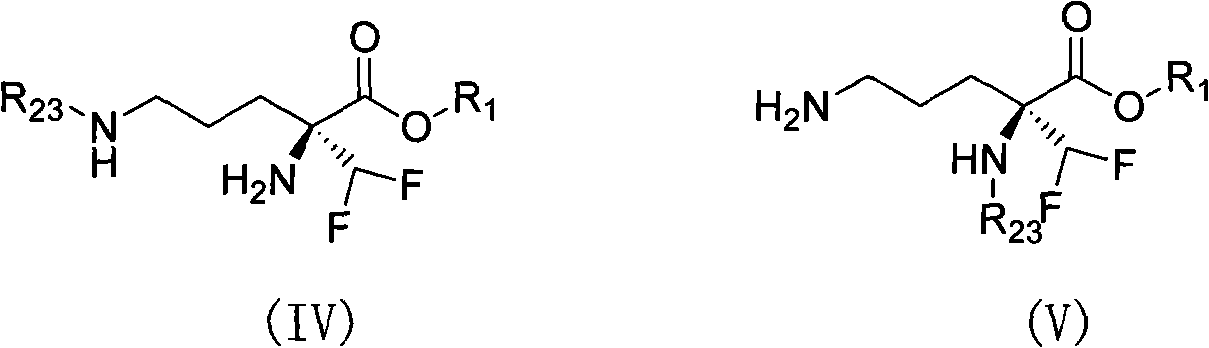
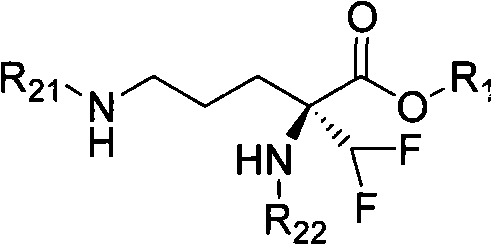
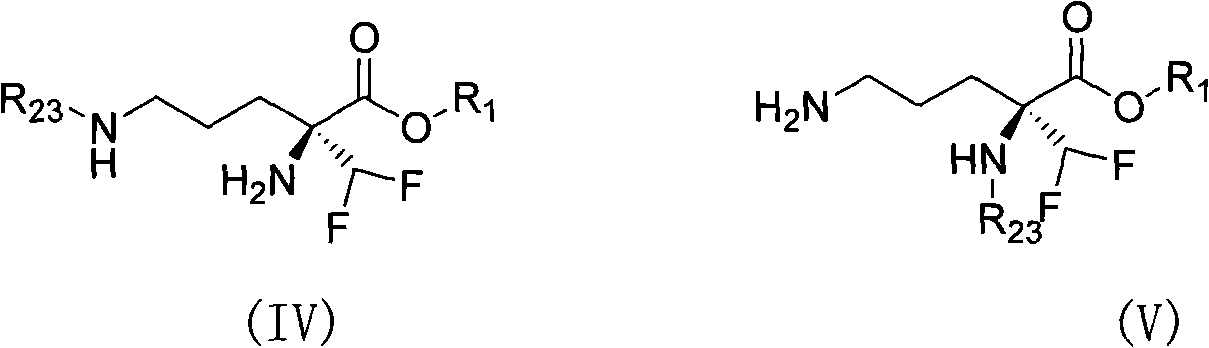Eflornithine prodrug and conjugate and using method thereof
A kind of eflunomine, drug technology, applied in the field of eflunomine prodrugs and conjugates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Example 1: Synthesis of Eflornithine-Aspirin Conjugate
[0154] The invention provides a method for synthesizing an eflornithine-NSAID conjugate. The examples shown below generally describe the synthesis of eflornithine analog-NSAID conjugates, and more specifically the synthesis of eflornithine-aspirin conjugates.
[0155]
Embodiment 2
[0156] Embodiment 2: Synthesis of Eflornithine Prodrug
[0157] This example generally provides the preparation method of eflornithine prodrug, more specifically phosphoramidate prodrug.
[0158]
Embodiment 3
[0159] Example 3: In Vitro Test of Caco-2 Cell Permeability of Eflornithine Conjugates or Eflornithine Prodrugs
[0160] Passive permeation of the eflornithine-NSAID conjugates or eflornithine prodrugs of the present invention is performed in vitro using methods well known in the art (see, e.g., Stewart, et al., Pharm. Res. ., 1995, 12, 693). For example, passive permeability is determined by testing the flux of eflornithine-NSAID conjugates or eflornithine prodrugs through cultured polarized cell monolayers (eg, Caco-2 cells). Caco-2 cells obtained through continuous culture (less than 28 passages) were transferred to Transwell polycarbonate filter at high concentration. Cells were stored in DMEM / 10% fetal bovine serum + 0.1 mM non-essential amino acids + 2 mM L-Gln, 5% CO2 / 95% O2, 37°C until the day of the experiment. Permeability studies were performed at pH 6.5 apically (in 50 mM MES buffer containing 1 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, 3 mM KCl, 1 mM NaH2PO4, 5 mM gluc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com