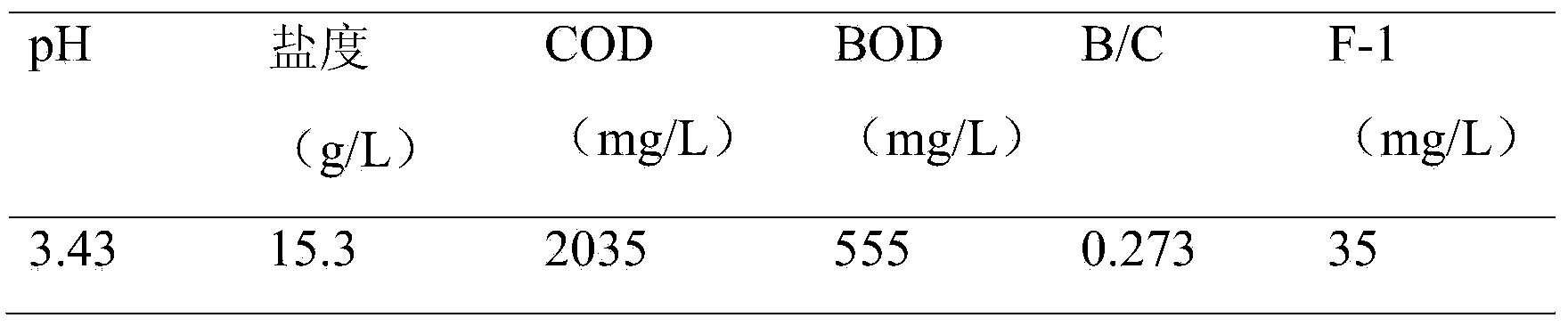Treatment process for fluorine chemical wastewater
A technology of chemical wastewater and treatment process, applied in the direction of water/sewage multi-stage treatment, water/sludge/sewage treatment, water pollutants, etc. Solve and affect the efficiency of the treatment process, etc., to achieve the effect of increasing the processing capacity, saving the cost of chemical dosing, and shortening the precipitation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
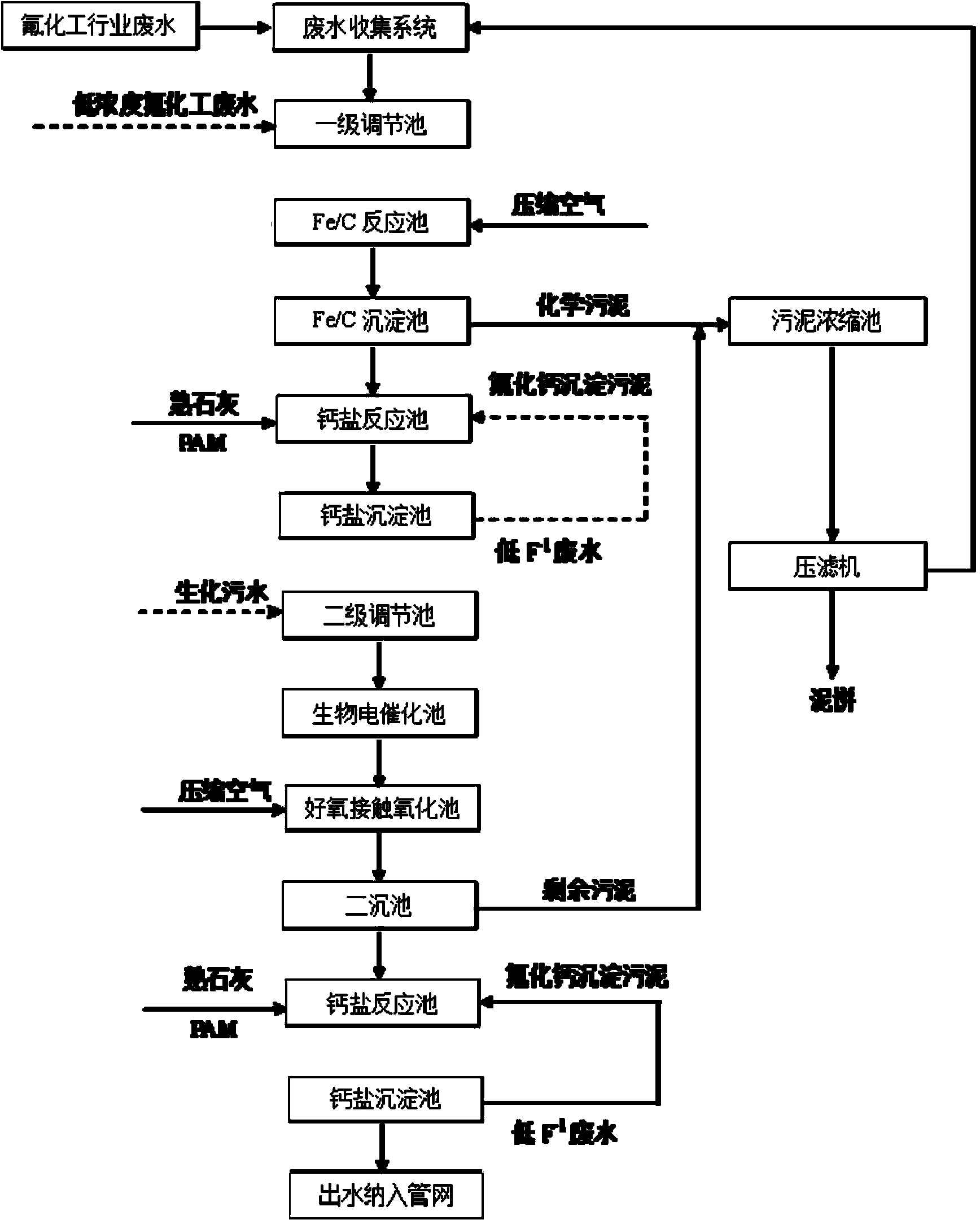
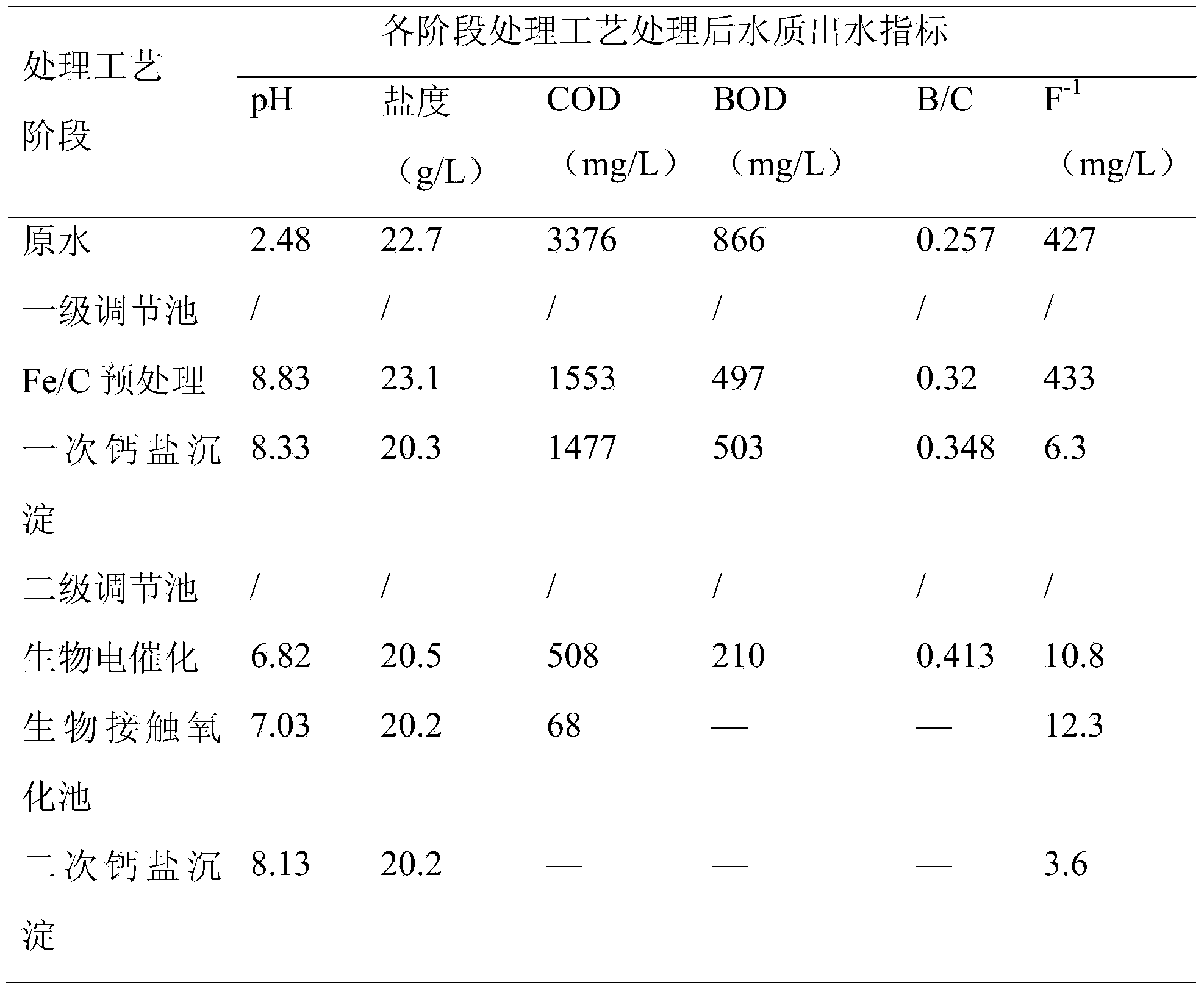
[0060] (1) The wastewater is pumped to the iron-carbon reaction tank, stirred with compressed air in the iron-carbon tank, and industrial waste acid or waste alkali is added through the online pH control dosing pump to maintain the pH value of the reaction in the tank; the iron-carbon reaction tank is laid Iron-carbon filler, the Fe / C of iron-carbon filler is 1:1; the liquid-solid volume ratio of iron-carbon filler to wastewater is 10:1; the hydraulic retention time of iron-carbon reaction tank is 4h;
[0061] (2) After the reaction, the wastewater flows to the iron-carbon sedimentation tank by itself, and the pH value of the wastewater is adjusted for flocculation and sedimentation. The precipitated sludge enters the sludge thickening tank to concentrate and then is filtered to remove water; the sedimentation time of the iron-carbon sedimentation tank is 1h;
[0062] (3) The supernatant of the precipitated wastewater in the iron-carbon sedimentation tank overflows to the first...
Embodiment 2
[0071] (1) The wastewater from the fluorine chemical industry enters the first-level adjustment pool for pH adjustment, and the low-concentration fluorine chemical wastewater in Table 2 is used for adjustment. The raw water and the low-concentration fluorine chemical wastewater are diluted and adjusted at a volume ratio of 1:4, and the pH of the wastewater is adjusted. value;
[0072] (2) The adjusted wastewater is pumped to the iron-carbon reaction tank, stirred with compressed air in the iron-carbon tank, and industrial waste acid or alkali is added through the online pH control dosing pump to maintain the pH value of the reaction in the tank; The iron-carbon filler is laid in the carbon reaction tank, and the Fe / C of the iron-carbon filler is 1:1; the liquid-solid volume ratio of the iron-carbon filler to the waste water is 10:1; the hydraulic retention time of the iron-carbon reaction tank is 5h; (3) Reaction Finally, the wastewater flows to the iron-carbon radial flow sed...
Embodiment 3
[0084] (1) The wastewater enters the first-level adjustment tank for pH adjustment, and the low-concentration fluorine chemical wastewater in Table 2 is used for adjustment, and the raw water and the low-concentration fluorine chemical wastewater are diluted and adjusted at a volume ratio of 1:9, and the pH value of the wastewater is adjusted;
[0085] (2) The adjusted wastewater is pumped to the iron-carbon reaction tank, stirred with compressed air in the iron-carbon tank, and industrial waste acid or waste alkali is added through the online pH control dosing pump to maintain the pH value of the reaction in the tank; The iron-carbon filler is laid in the reaction tank, and the Fe / C of the iron-carbon filler is 1:1; the liquid-solid volume ratio of the iron-carbon filler to the waste water is 10:1; the hydraulic retention time of the iron-carbon reaction tank is 6h;
[0086] (3) After the reaction, the wastewater flows to the iron-carbon radial flow sedimentation tank by itsel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical oxygen demand (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com