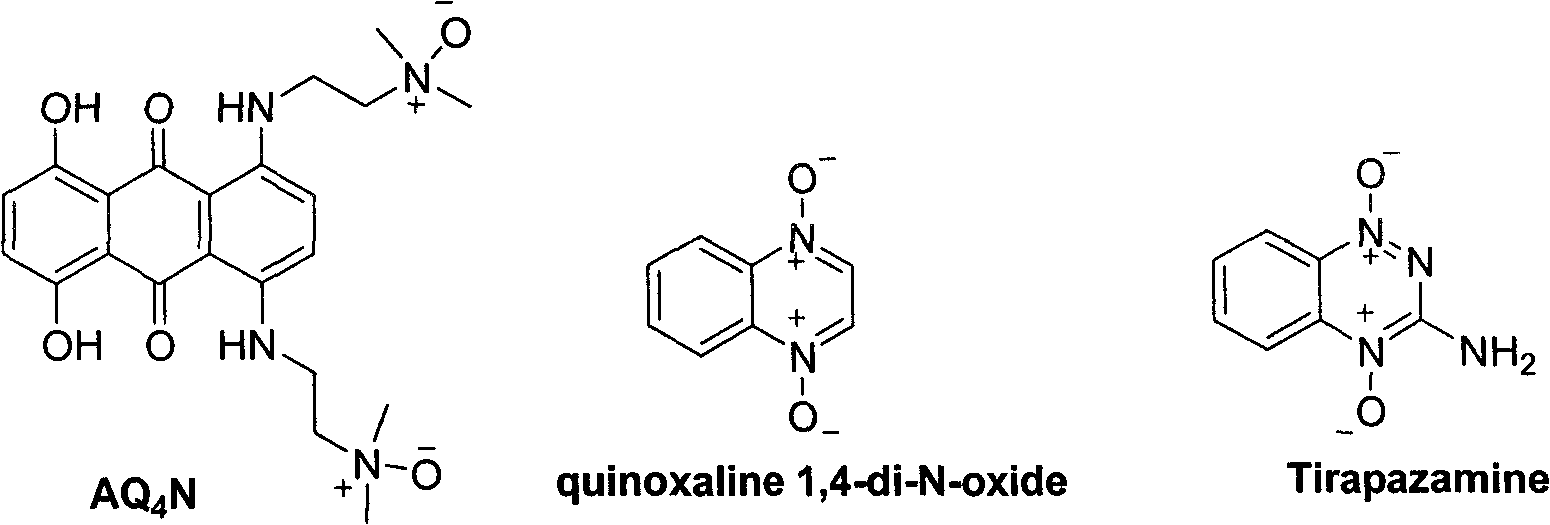
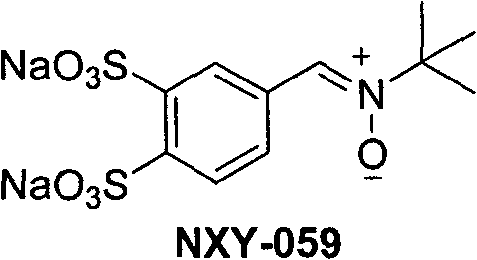
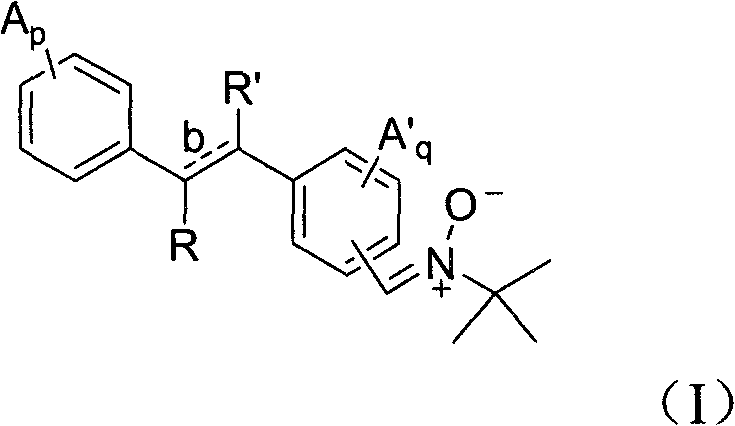
Phenyl nitrone compounds containing stilbene sections and application thereof
The technology of a compound and nitrone, which is applied in the preparation of anti-tumor drugs, the preparation of the compounds, and the field of neuroprotective agents, can solve problems such as hindering the clinical application of targeted anti-tumor drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 according to route 1 synthetic formula (I) compound
[0052] 1.1 Synthesis of compound (2)
[0053] Add methyl bromomethylbenzoate 1 (5 g, 0.022 mol) and triethyl phosphite (5.4 g, 0.033 mol) into 50 mL of toluene, heat to 105 ° C and reflux for 24 hours, cool down to room temperature, and remove toluene by rotary evaporation , to obtain yellowish liquid 2 (5.8 g, 93%). 1 HNMR (400MHz, CDCl 3 )δ1.11-1.15(m, 6H), 3.03-3.05(m, 2H), 3.88(s, 3H), 4.07-4.10(m, 4H), 7.17(m, 2H), 7.85(m, 2H) ; MS(EI) m / z 166[M] + .
[0054] 1.2 Synthesis of compound (3)
[0055] Sodium methoxide (2.5g, 0.046mol) was dissolved in 50mL ether, compound 2 (5.8g, 0.035mol) and 3,5-dimethoxybenzaldehyde (2.5g, 0.015mol) were dissolved in 25mL ether and slowly Add dropwise into the sodium methoxide solution, add about 0.5 hours, stir at room temperature for 20 minutes, plate chromatography (TLC) monitor the reaction is complete, add 70mL dichloromethane, 100mL water, neutralize ...
Embodiment 2
[0062] Embodiment 2 screening compound by Caliper method
[0063] In this example, 12 compounds were screened against the in vitro kinases EGFR, HER2, PDGFRa, PDGFRb and SRC using the Caliper method, and the compound staurosporine was used as a standard control, and each compound was diluted to 10 concentration points for repeated well detection. The reaction conditions and results are shown in Table 1 and Table 2, respectively.
[0064] Table 1
[0065] Kinase
Enzyme concentration (nM)
ATP concentration (μM)
With or without MnCl 2
Reaction time
EGFR
8
2.3
Contains 10mM MnCl 2
1 hour
HER2
18
15
Contains 10mMMnCl 2
1 hour
3.5
134
5 hours
PDGFRb
6
38
5 hours
SRC
1
36
1 hour
[0066] Table 2
[0067]
Embodiment 3
[0068] Embodiment 3 compound influences on cell proliferation activity
[0069] The effects of the above compounds 6-17 on the proliferation of HepG2, A549, BGC-823 and HCT116 cell lines were detected by MTT assay.
[0070]10 mM compounds were prepared in DMSO and diluted 1:5. Each compound was detected from a final concentration of 50 μM. 0.5 μl was pipetted from the corresponding compound plate and added to a cell culture plate, and incubated in a 37° C. incubator for 72 hours. Then the cell morphology was observed under an inverted microscope, and 20 μl of 5 mg / ml MTT solution prepared from sterile PBS was added to each well. Incubate in a 37°C incubator for 5 hours, add 100 μl triple dissolving solution, and dissolve overnight in a 37°C biochemical incubator. Absorbance values were detected using Flexstation3. Record the IC50 value obtained from the analysis. Table 3 shows the inhibitory rates of the above-mentioned 12 compounds on cell line proliferation.
[0071] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com