Medical composite containing pirfenidone and preparation method thereof
The technology of a kind of pirfenidone and composition is applied in the field of pirfenidone medicinal composition, which can solve the problems that pirfenidone has not been listed on the market, etc., so as to achieve the effect of drug effect, fast dissolution rate, and reduce the discomfort of human body. Tolerance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: preparation of pirfenidone tablet (0.2g / sheet)
[0026] Prescription: Pirfenidone 200g
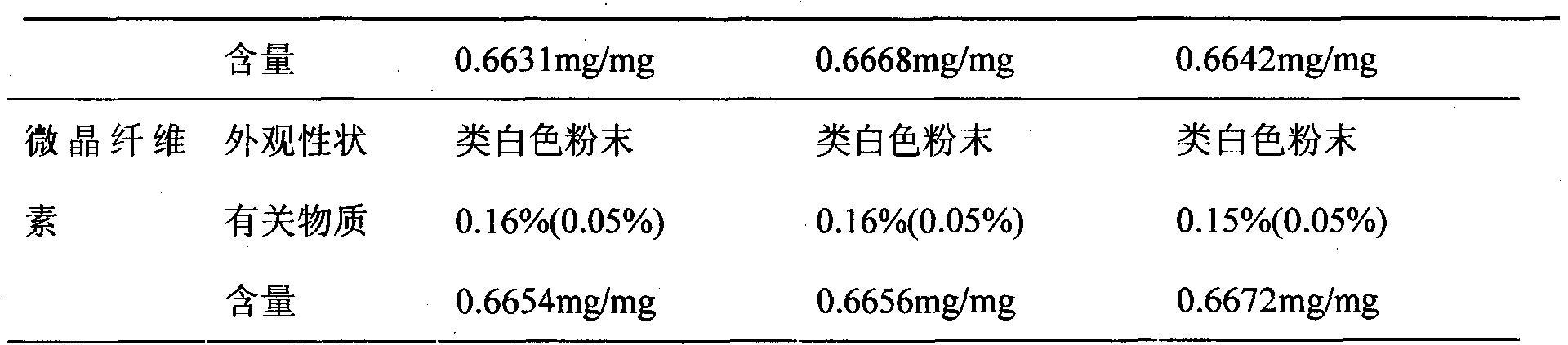
[0027] Microcrystalline Cellulose 56g
[0028] Sodium carboxymethyl starch 20g
[0029] Hypromellose 6g
[0031]
[0032] 1000 pieces
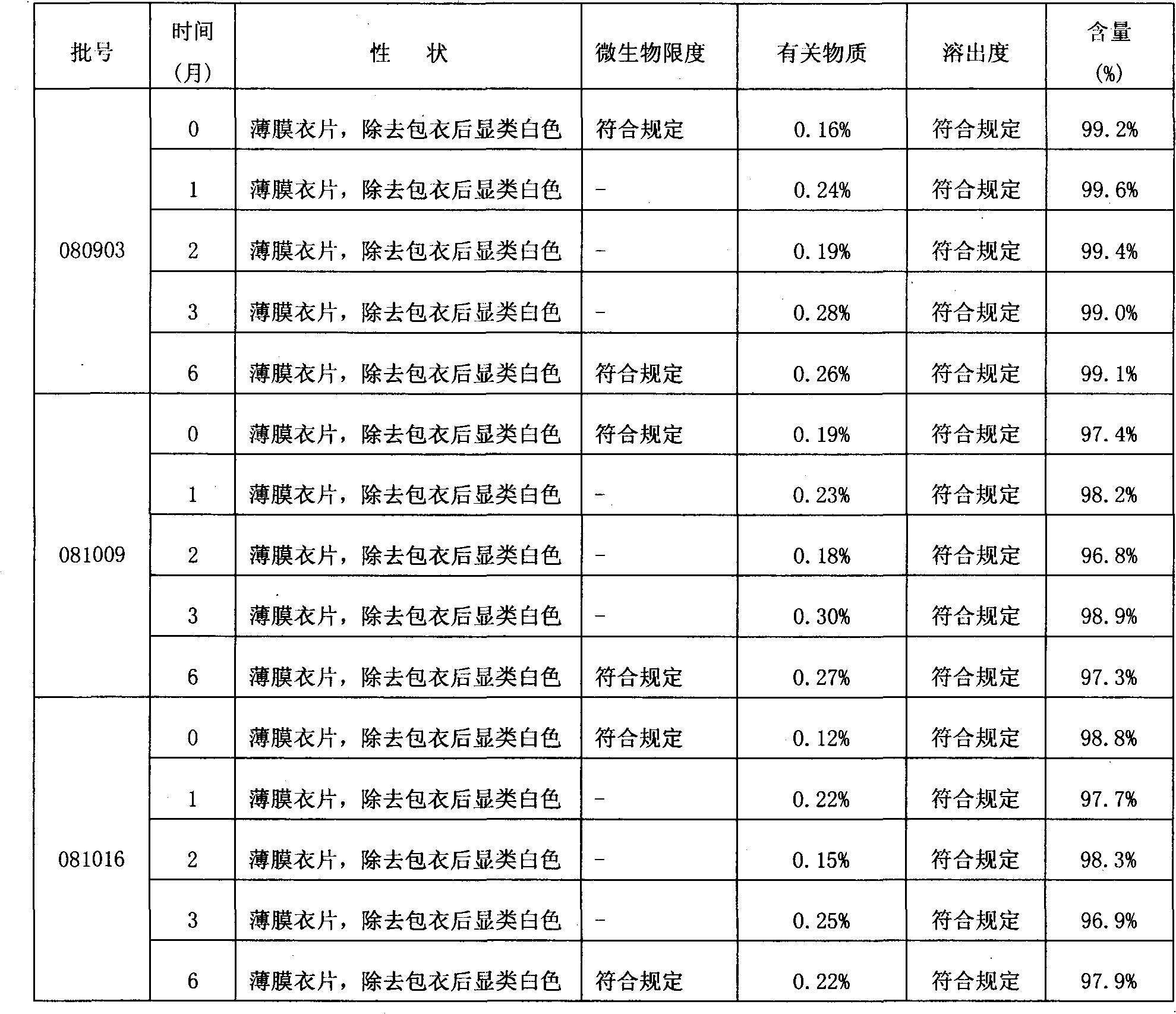
[0033] Take by weighing 20g sodium carboxymethyl starch and the pirfenidone and microcrystalline cellulose of prescription quantity and pulverize respectively, cross 80 mesh sieves respectively for subsequent use; The raw and auxiliary materials that will pulverize are taken by prescription quantity and mix evenly standby; Make soft material, granulate with 20 mesh sieve, dry at 50-60°C; pass through 20 mesh sieve for granulation, add prescription amount of magnesium stearate, and finally mix; measure the content of intermediates, determine the weight of the tablet, and compress the tablet; The plain tablet is coated (the we...
Embodiment 2
[0034] Embodiment 2: preparation of pirfenidone tablet (0.2g / sheet)
[0035] Prescription: Pirfenidone 200g
[0036] Microcrystalline Cellulose 56g
[0037] Sodium carboxymethyl starch 10g+10g
[0038] Hypromellose 6g
[0040]
[0041] 1000 pieces
[0042] Weigh 10g of sodium carboxymethyl starch and place it at 105°C for drying for 5 hours for later use; weigh 10g of sodium carboxymethyl starch and the prescribed amount of pirfenidone and microcrystalline cellulose and pulverize them respectively, and pass through 80 mesh sieves respectively for later use; The raw and auxiliary materials are weighed according to the prescription amount and mixed evenly for later use; add distilled water to make the material into soft material, granulate with a 20-mesh sieve, and dry at 50-60°C; Sodium carboxymethyl starch, final mixing; measure the content of interme...
Embodiment 3
[0043] Embodiment 3: preparation of pirfenidone tablet (0.4g / sheet)
[0044] Prescription: Pirfenidone 400g
[0045] Microcrystalline Cellulose 112g
[0046] Sodium Carboxymethyl Starch 20g+20g
[0047] Hypromellose 12g
[0049]
[0050]1000 pieces
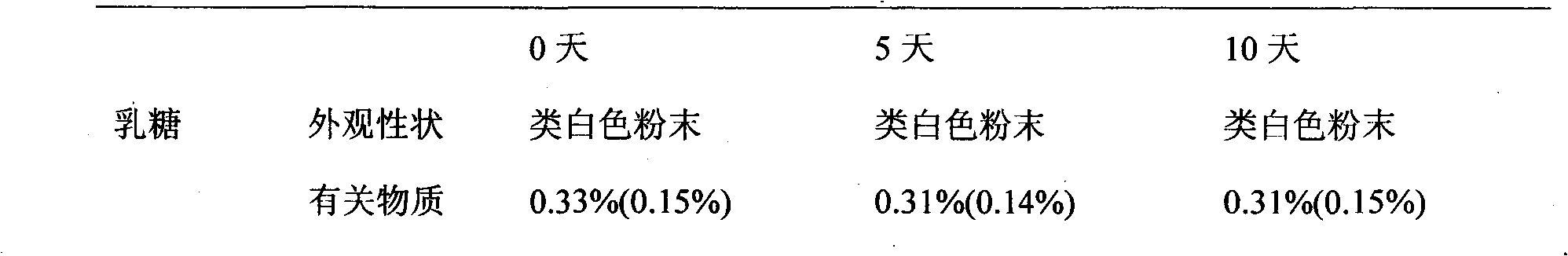
[0051] Weigh 20g of sodium carboxymethyl starch and place it at 105°C for drying for 5 hours for later use; weigh 20g of sodium carboxymethyl starch and the prescribed amount of pirfenidone and lactose and pulverize them respectively, and pass through 80 mesh sieves respectively for later use; the crushed raw and auxiliary materials Weigh and mix evenly according to the prescription amount for later use; add distilled water to make the material soft material, granulate with a 20-mesh sieve, and dry at 50-60°C; pass through a 20-mesh sieve for granulation, add the prescription amount of magnesium stearate and 20g carboxymethyl Sodium sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com