Antifreeze polypeptide prepared by enzymolysis of cow leather collagen by alkali protease
An antifreeze polypeptide and protease technology, applied in the biological field, can solve the problems of small quantity and limited application prospects of large laws.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The antifreeze activity detection system for preparing antifreeze polypeptide of the present invention adopts a combined cold-heat-stage system, multi-fold microscope and automatic camera system to construct an antifreeze activity inhibition ice crystal growth detection system platform. The cryogenic environment during operation was maintained with liquid nitrogen. Simulate temperature fluctuations of products during cryogenic storage. Through the system program setting, set the temperature fluctuation of the sample and repeat it continuously for 7 to 25 cycles, connect a multi-fold microscope and a real-time photographic recording system, and record the real-time ice crystal growth of the sample during the cold-hot cycle.
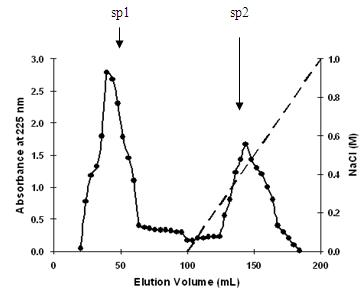
[0035] Sephadex G-50 molecular sieves, Sepharose SP C-25 ion exchange chromatography, RP-HPLC reversed-phase high-performance liquid chromatography, dialysis and other separation and purification methods are used to achieve efficient separation and ...
Embodiment 1
[0040] Weigh 1.65 g bovine hide collagen and dissolve it in 6 ml Mili-Q water, then adjust its pH to 9.0 with 2 mol / L NaOH. First heat the solution to 45°C in a water bath, then add the corresponding amount of enzyme according to the enzyme-substrate ratio of 1:15, and the enzymatic hydrolysis time is 30 minutes. Then inactivate the enzyme in a boiling water bath for 10 minutes, then centrifuge at 14000 rpm for 10 minutes after cooling, and collect the supernatant for later use.
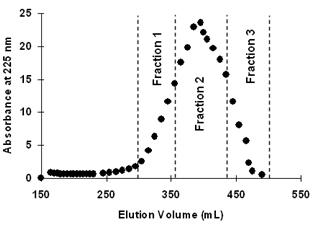
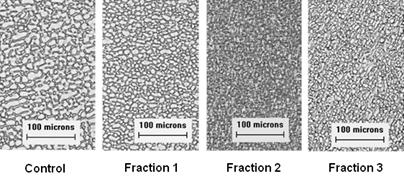
[0041] The supernatant was separated by Sephadex G-50 gel chromatography (length 100cm, diameter 2.6cm), the eluent was deionized water, the flow rate was 2mL / min, and the elution peak was measured at 225nm, see figure 1 . Peaks were collected and assayed for antifreeze activity (eg figure 2 shown). From figure 1 It can be seen that Fraction2 has the best antifreeze activity. Statistical analysis shows that the average diameter of ice crystals is 5.02um, which is significantly smaller than F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com