Interpenetrated reticular proton exchange membrane, forming method thereof and proton exchange membrane fuel cell
A technology of proton exchange membrane and network, which is applied in the composition field of improving the dimensional stability of proton exchange membrane to achieve the effect of improving physical properties, optimal glass transition temperature and dimensional stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
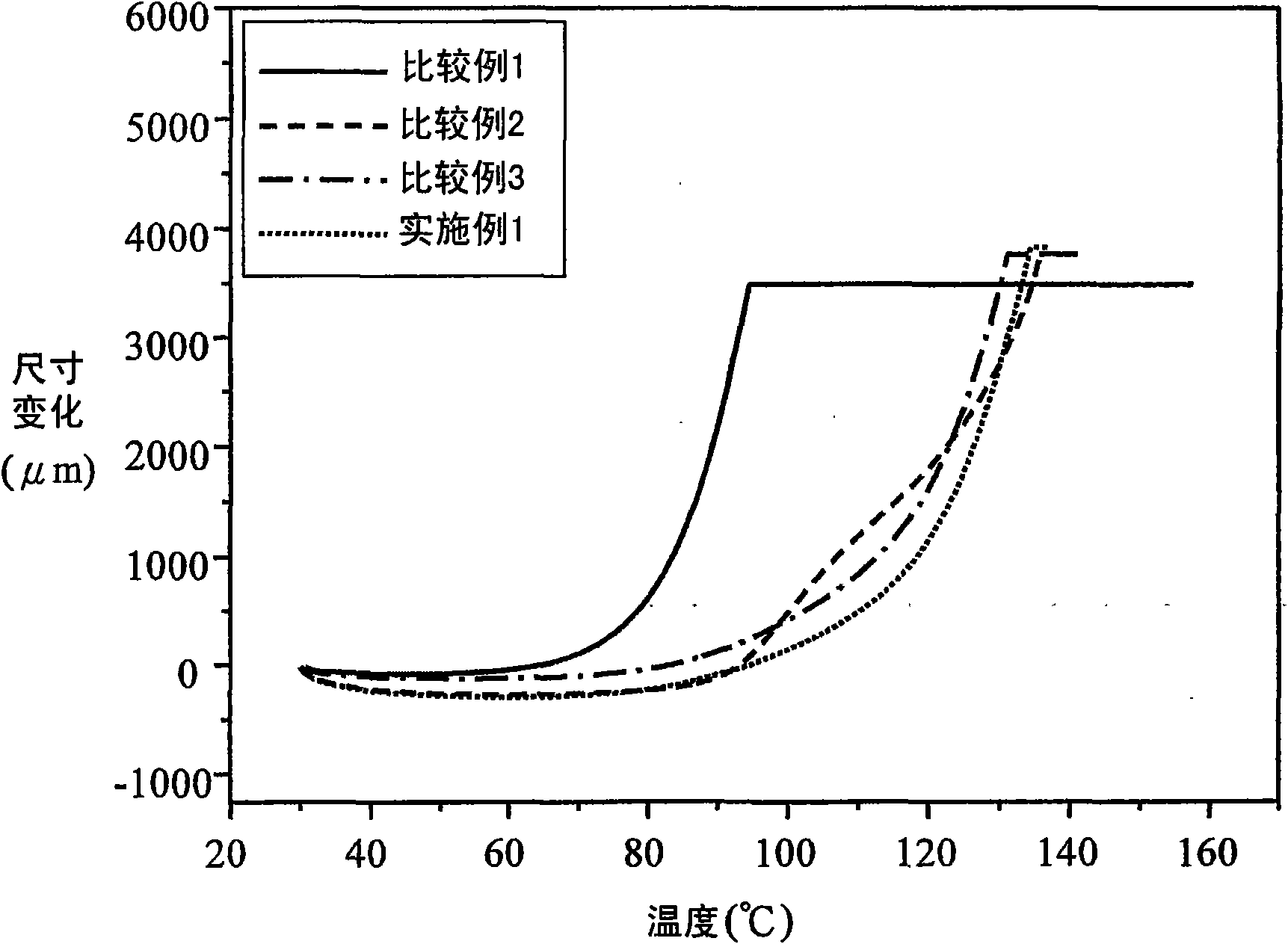
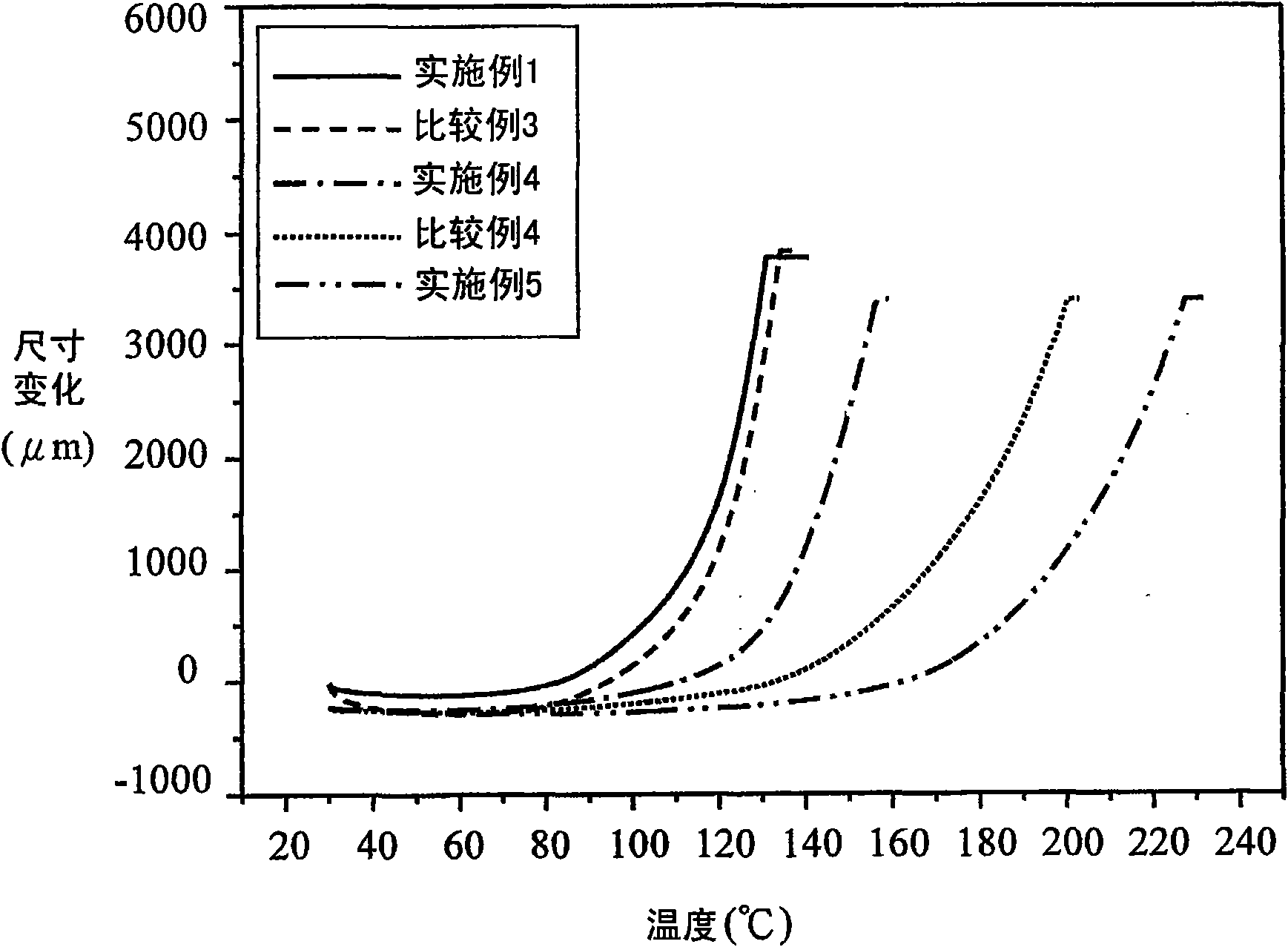
Embodiment 1
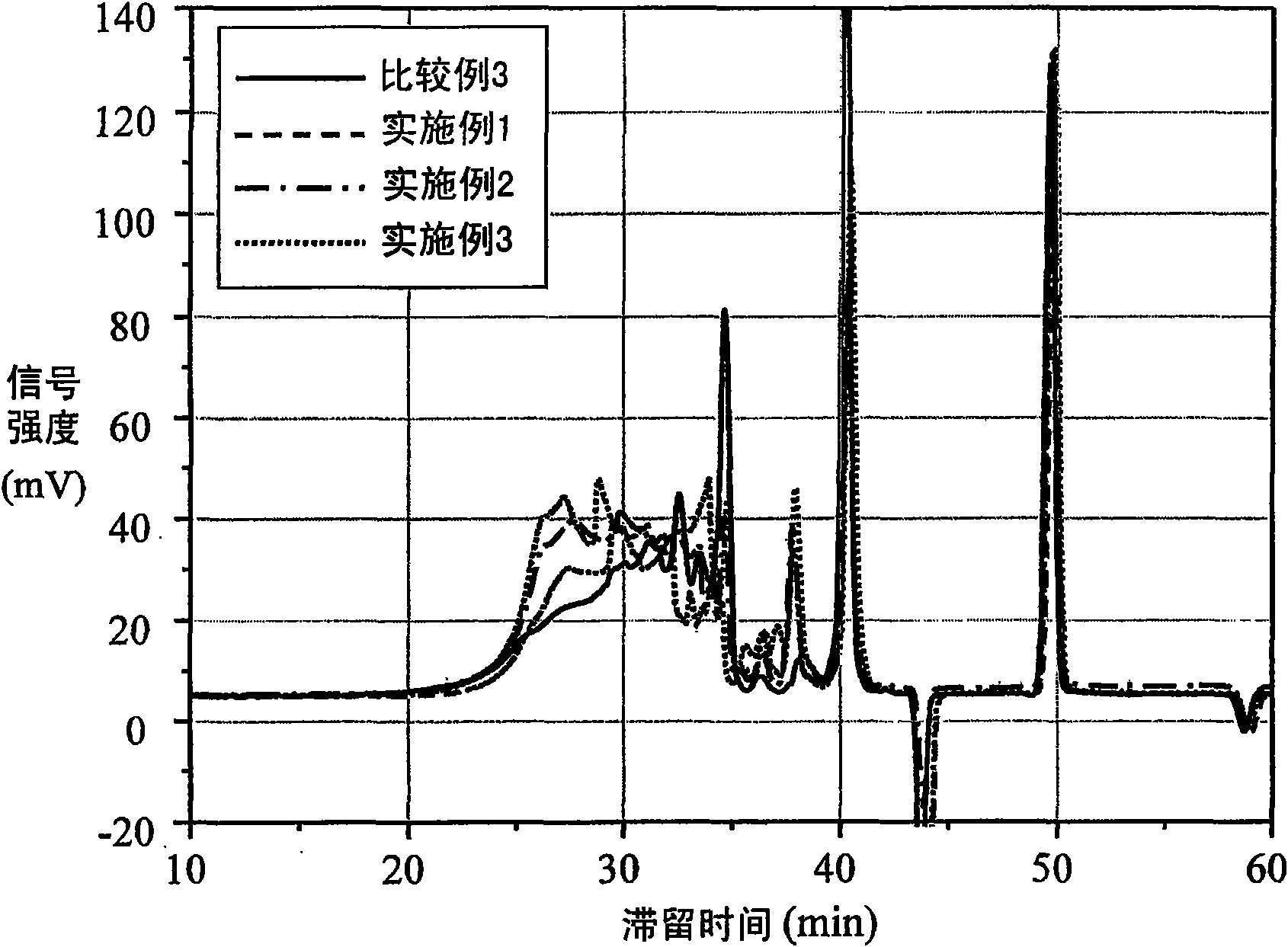
[0043] Get the polymaleimide of 16.967g formula 1 (BMI2300, purchased from KAIWAKSEI, it is the mixture of polymaleimide, wherein n=0 accounts for 60mole%, n=1 accounts for 23mole%, n=2 accounts for 10mole %, n=3 accounts for 7 mole%) and barbituric acid (purchased from ALDRICH) in a molar ratio of 2:1, after adding the solvent γ-butyrolactone (γ-butyrolactone, GBL), heated to 105 ° C after the reaction After 5 hours, the reaction produces super-branched polymers. Measure the above-mentioned super-branched polymer with gel permeation liquid chromatography (GPC), and its spectrum is as follows figure 1 shown. The residence time of the highly branched polymer is about 26 to 40 minutes, the number average molecular weight (Mn) is 10204, the weight average molecular weight is 29981, and the polymerization distribution index (Mw / Mn) is 2.93.
[0044] (Formula 1)
[0045] Next, take 30 g of Nafion aqueous solution (DE2020CS purchased from DuPont), add 18 g of DMAc solution, hea...
Embodiment 2
[0049] Get 16.967g polymaleimide of formula 1 (BMI2300, purchased from KAIWAKSEI, its composition is the same as embodiment 1) and barbituric acid (purchased from ALDRICH) and mix with molar ratio 2: 1, add solvent γ-butyl Lactone (γ-butyrolactone, GBL) was heated to 115°C and reacted for 5 hours to form a super branched polymer. Measure the above-mentioned super-branched polymer with gel permeation liquid chromatography (GPC), and its spectrum is as follows figure 1 shown. The residence time of the highly branched polymer is about 26 to 40 minutes, the number average molecular weight (Mn) is 16419, the weight average molecular weight is 67208, and the polymerization distribution index (Mw / Mn) is 4.09.
[0050] (Formula 1)
[0051] Next, take 30 g of Nafion aqueous solution (DE2020CS purchased from DuPont), add 18 g of DMAc solution, heat at 60° C., and replace the water and alcohols in the Nafion aqueous solution with DMAc.
[0052] After adding the above super branched ...
Embodiment 3
[0055] Get 16.967g polymaleimide of formula 1 (BMI2300, purchased from KAIWAKSEI, its composition is the same as embodiment 1) and barbituric acid (purchased from ALDRICH) and mix with molar ratio 2: 1, add solvent γ-butyl Lactone (γ-butyrolactone, GBL) was heated to 125°C and reacted for 5 hours to form a super-branched polymer. Measure the above-mentioned super-branched polymer with gel permeation liquid chromatography (GPC), and its spectrum is as follows figure 1 shown. The residence time of the highly branched polymer is about 26 to 40 minutes, the number average molecular weight (Mn) is 15602, the weight average molecular weight is 65689, and the polymerization distribution index (Mw / Mn) is 4.18.
[0056] (Formula 1)
[0057] Next, take 30 g of Nafion aqueous solution (DE2020CS purchased from DuPont), add 18 g of DMAc solution, heat at 60° C., and replace the water and alcohols in the Nafion aqueous solution with DMAc.
[0058] After adding the above super branched ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com