Silver nanoparticles with function of identifying chiral isomer, preparation method thereof and application thereof
A technology of nano-silver particles and chiral isomers, applied in chemical instruments and methods, measurement of color/spectral characteristics, and analysis through chemical reactions of materials, etc., can solve the problems of narrowing the application range and achieve a wide range of use , good operability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
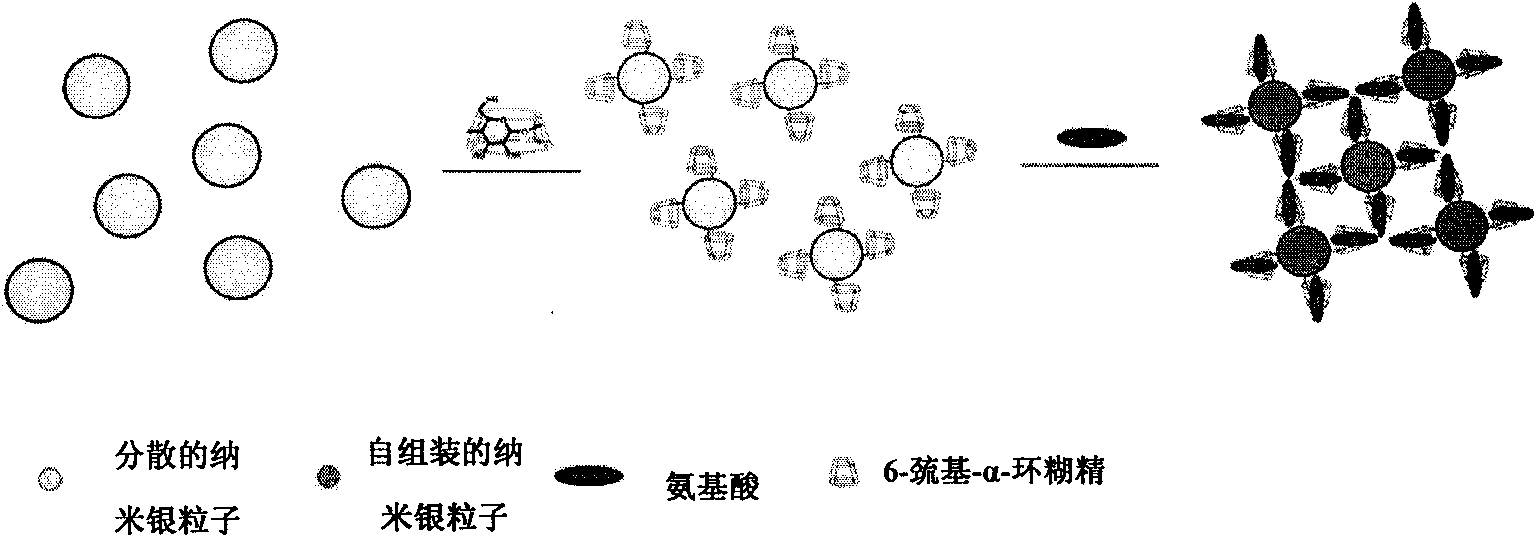
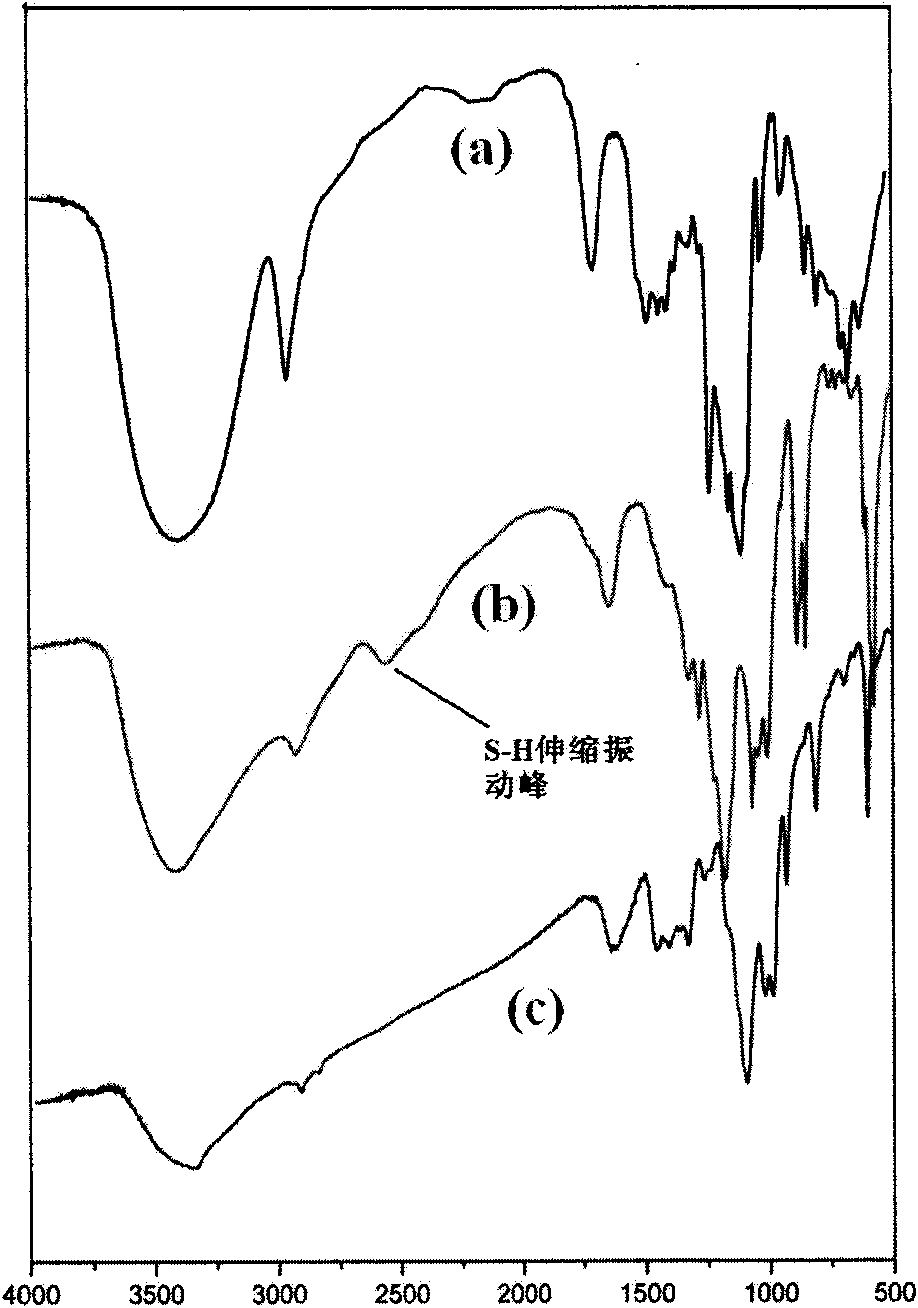
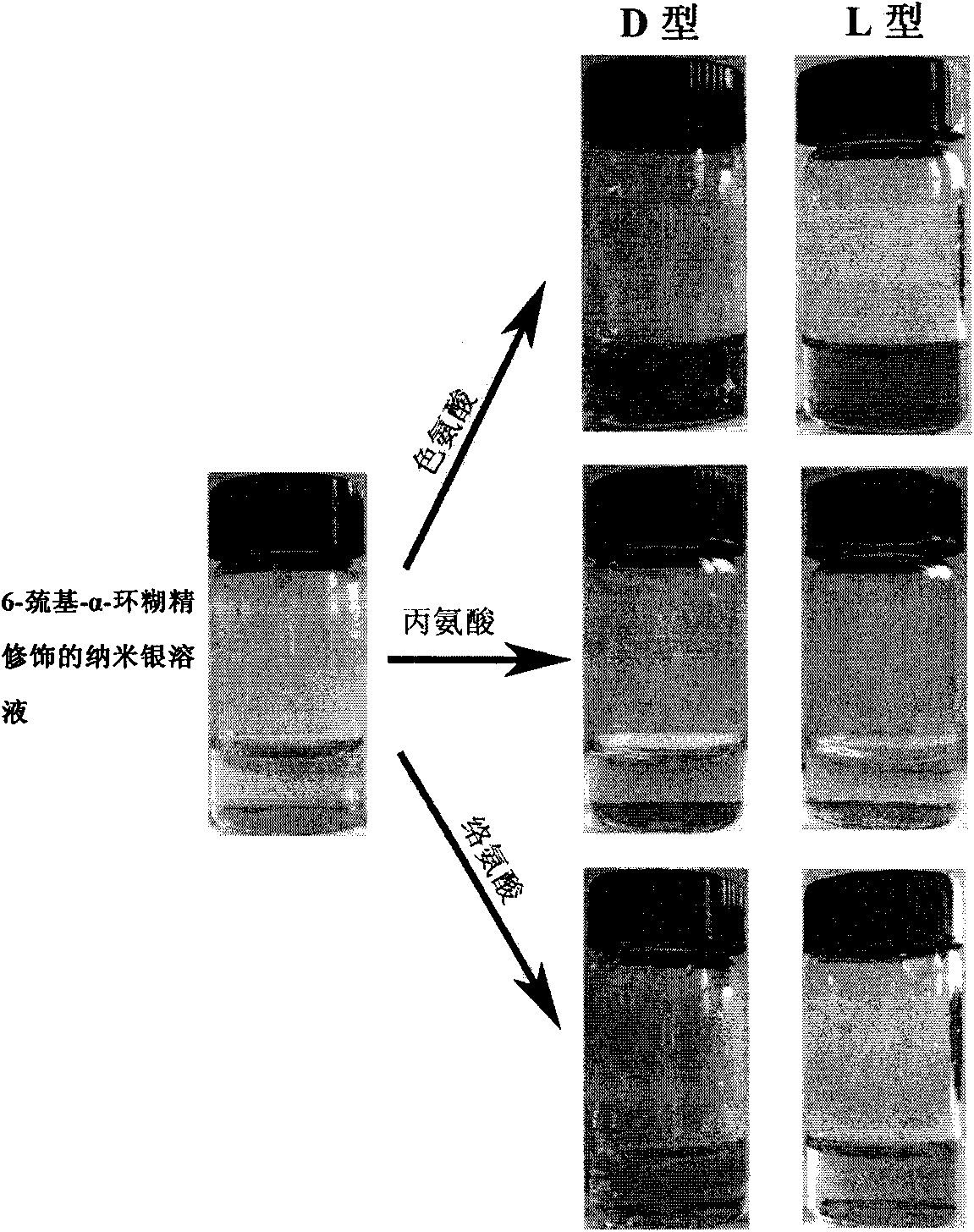
[0029] Embodiment 1, preparation 6-mercapto-alpha-cyclodextrin modified silver nanoparticles
[0030] 1) Weigh 21g of triphenylphosphine and 20.2g of iodine and dissolve in 80ml of N,N-dimethylformamide, add 4.32g of α-cyclodextrin after fully dissolved, and react at 80°C for 15 hours. Concentrate in vacuo to 40ml, add 30ml of sodium methoxide-methanol solution with a concentration of 3mol / L, and adjust the pH value to 9.5. The system was cooled to 25°C, and after stirring for 30 minutes, the system was precipitated with 800 ml of methanol, the precipitate was washed with methanol, and dried in vacuum to obtain 6-iodo-α-cyclodextrin.
[0031] 2) Dissolve 0.965 g of 6-iodo-α-cyclodextrin in 10 ml of N,N-dimethylformamide, and add 0.301 g of thiourea. Stir and heat up to 70°C, and react for 19 hours under nitrogen protection. After the reaction was completed, N,N-dimethylformamide was distilled off under reduced pressure to obtain 0.914 g of compound a as a yellow oil.
[003...
Embodiment 2
[0036] Embodiment 2, preparation 6-mercapto-α-cyclodextrin modified silver nanoparticles
[0037] 1) Weigh 23g of triphenylphosphine and 21.2g of iodine and dissolve in 85ml of N,N-dimethylformamide, add 4.52g of α-cyclodextrin after fully dissolved, and react at 83°C for 19 hours. Concentrate in vacuo to 42ml, add 31ml of sodium methoxide-methanol solution with a concentration of 3.2mol / L, and adjust the pH value to 10. The system was cooled to 25°C, and after stirring for 35 minutes, the system was precipitated with 1000 ml of methanol, the precipitate was washed with methanol, and dried in vacuum to obtain 6-iodo-α-cyclodextrin.
[0038] 2) 1 g of 6-iodo-α-cyclodextrin was dissolved in 15 ml of N,N-dimethylformamide, and 0.4 g of thiourea was added. Stir and heat up to 70°C, and react for 20 hours under nitrogen protection. After the reaction was completed, N,N-dimethylformamide was distilled off under reduced pressure to obtain 0.963 g of compound a as a yellow oil.
[...
Embodiment 3
[0041] Example 3, preparation of silver nanoparticles modified by 6-mercapto-α-cyclodextrin
[0042] 1) Weigh 20g of triphenylphosphine and 19.2g of iodine and dissolve in 75ml of N,N-dimethylformamide. After fully dissolving, add 4.12g of α-cyclodextrin and react at 83°C for 13 hours. Concentrate in vacuo to 38ml, add 28ml of sodium methoxide-methanol solution with a concentration of 2.9mol / L, and adjust the pH value to 9. The system was cooled to 25° C., stirred for 29 minutes, and precipitated with 750 ml of methanol. The precipitate was washed with methanol and dried in vacuum to obtain 6-iodo-α-cyclodextrin.
[0043] 2) Dissolve 0.9 g of 6-iodo-α-cyclodextrin in 5 ml of N,N-dimethylformamide, and add 0.2 g of thiourea. Stir and heat up to 65°C, and react for 18 hours under nitrogen protection. After the reaction was completed, N,N-dimethylformamide was distilled off under reduced pressure to obtain 0.875 g of compound a as a yellow oil.
[0044] 3) Add 45 ml of deioni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com