Combined medicament of omeprazole sodium
A technology of omeprazole sodium and medicine, applied in the directions of medicine combination, medicine formula, non-active ingredient medical preparations, etc., can solve problems such as adverse reaction liver, allergic reaction, damage reaction, etc., so as to reduce liver damage and avoid Allergic reaction, the effect of improving inner quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
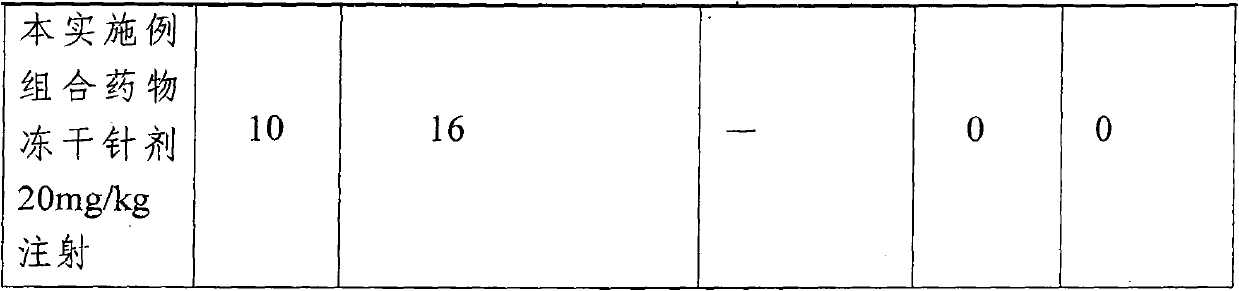
[0027] The omeprazole sodium combination medicine provided by the present embodiment comprises the following medicinal ingredients in parts by weight:
[0028] Omeprazole sodium: 17.3
[0029] Reduced Glutathione 8.7
[0030] Sodium glutamate 43.5
[0031] The preparation method of above-mentioned combination medicine is as follows:
[0032] (1). Take water for injection whose weight is 50 to 125 times of the weight of the omeprazole sodium, and dissolve the omeprazole sodium, reduced glutathione and sodium glutamate respectively under stirring Completely, get a solution of the drug.
[0033] (2). The solution obtained by the membrane ultrafiltration step (1) with a molecular weight cut-off of 8000D, the filtrate is ultrafiltered by a membrane with a molecular weight cut-off of 1500D, and the filtrate is adjusted to a pH value of 7.2 to 7.6 with 8% sodium hydroxide solution.
[0034] (3). The solution obtained in step (2) was sterilized by moist heat at 121° C. for 20 minu...
Embodiment 2
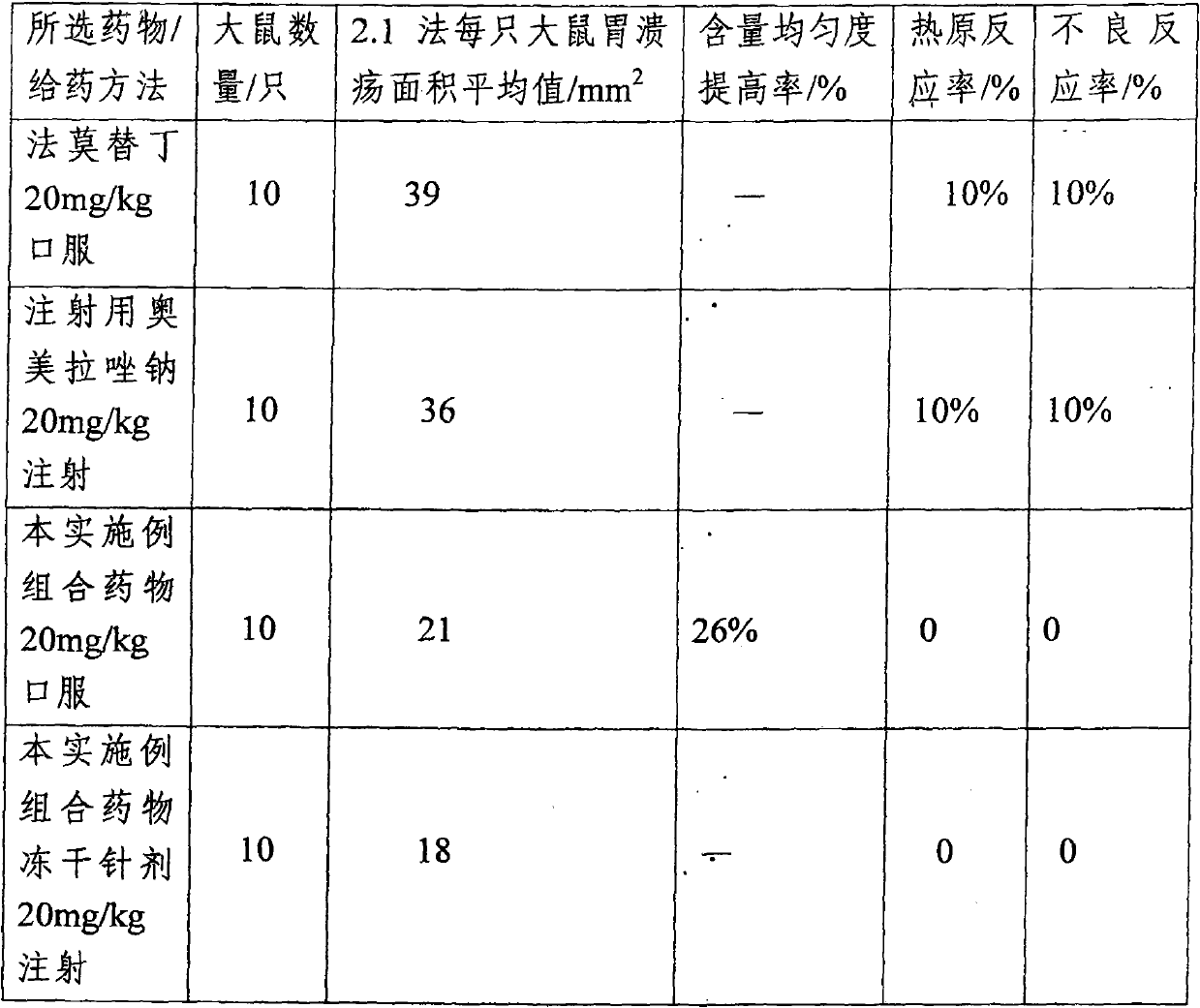
[0052] The omeprazole sodium combination medicine provided by the present embodiment comprises the following medicinal ingredients in parts by weight:
[0053] Omeprazole sodium: 34.8
[0054] Reduced Glutathione 13.0
[0055] Sodium glutamate 52.2
[0056] The preparation method of above-mentioned omeprazole sodium combination medicine is:
[0057] (1). Take water for injection whose weight is 50 to 125 times of the weight of the omeprazole sodium, and dissolve the omeprazole sodium, reduced glutathione and sodium glutamate respectively under stirring Completely, get a solution of the drug.
[0058](2). Be the solution that the membrane ultrafiltration step (1) that molecular weight cut off is 8000D obtains, and filtrate is the membrane ultrafiltration of 1500D with molecular weight cut off, and filtrate is regulated pH value to 7.2~7.6 with 8% sodium hydroxide solution.
[0059] (3). The solution obtained in step (2) was sterilized by moist heat at 121° C. for 20 minutes...
Embodiment 3
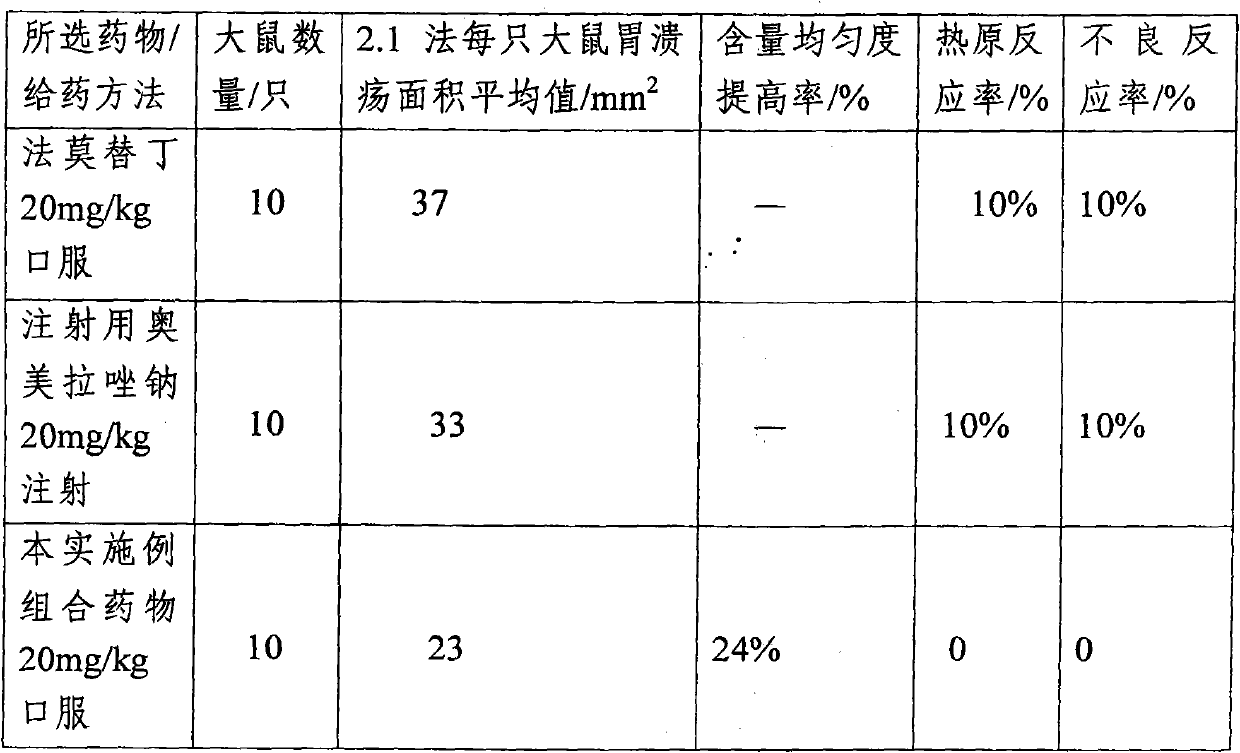
[0077] The omeprazole sodium combination medicine provided by the present embodiment comprises the following medicinal ingredients in parts by weight:
[0078] Omeprazole sodium: 29.1
[0079] Reduced Glutathione 10.2
[0080] Sodium glutamate 46.3
[0081] The preparation method of above-mentioned omeprazole sodium combination medicine is:
[0082] (1). Take water for injection whose weight is 50 to 125 times of the weight of the omeprazole sodium, and dissolve the omeprazole sodium, reduced glutathione and sodium glutamate respectively under stirring Completely, get a solution of the drug.
[0083] (2). The solution obtained by the membrane ultrafiltration step (1) with a molecular weight cut-off of 8000D, the filtrate is ultrafiltered by a membrane with a molecular weight cut-off of 1500D, and the filtrate is adjusted to a pH value of 7.2 to 7.6 with 8% sodium hydroxide solution.
[0084] (3). The solution obtained in step (2) was sterilized by moist heat at 121° C. for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com