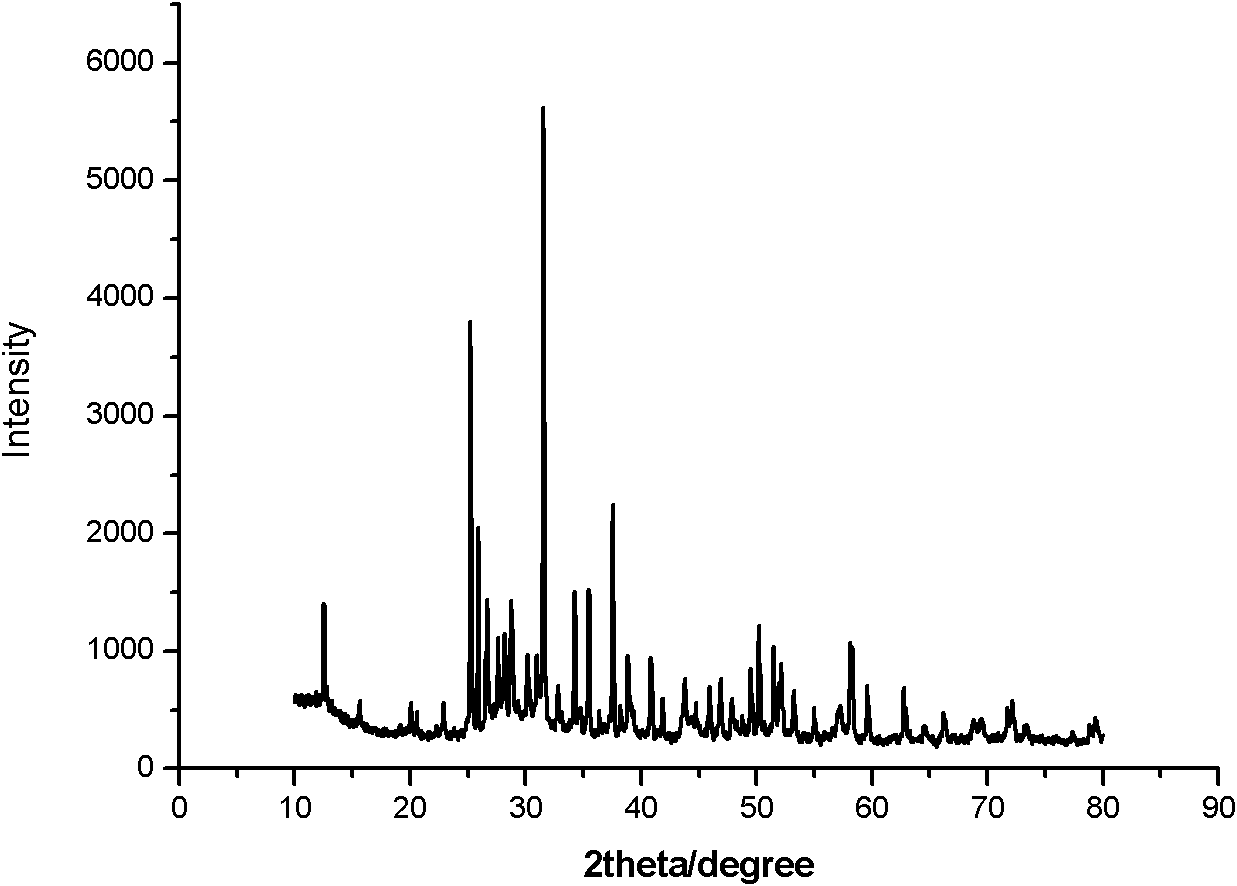
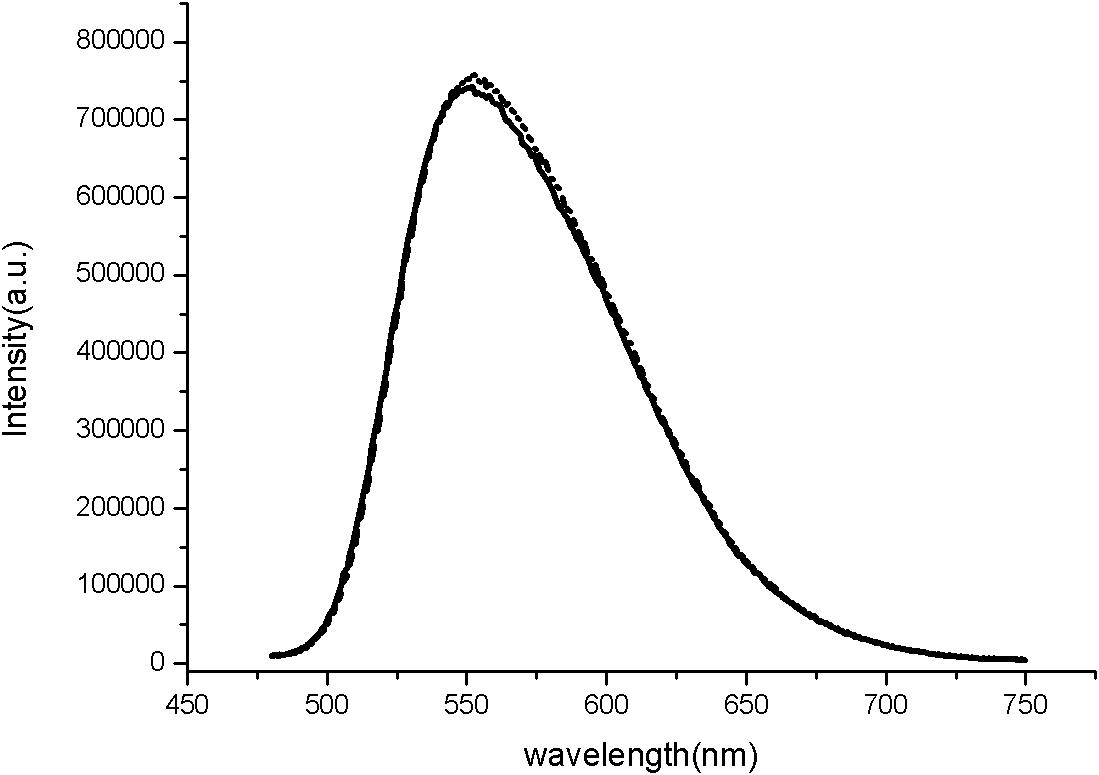
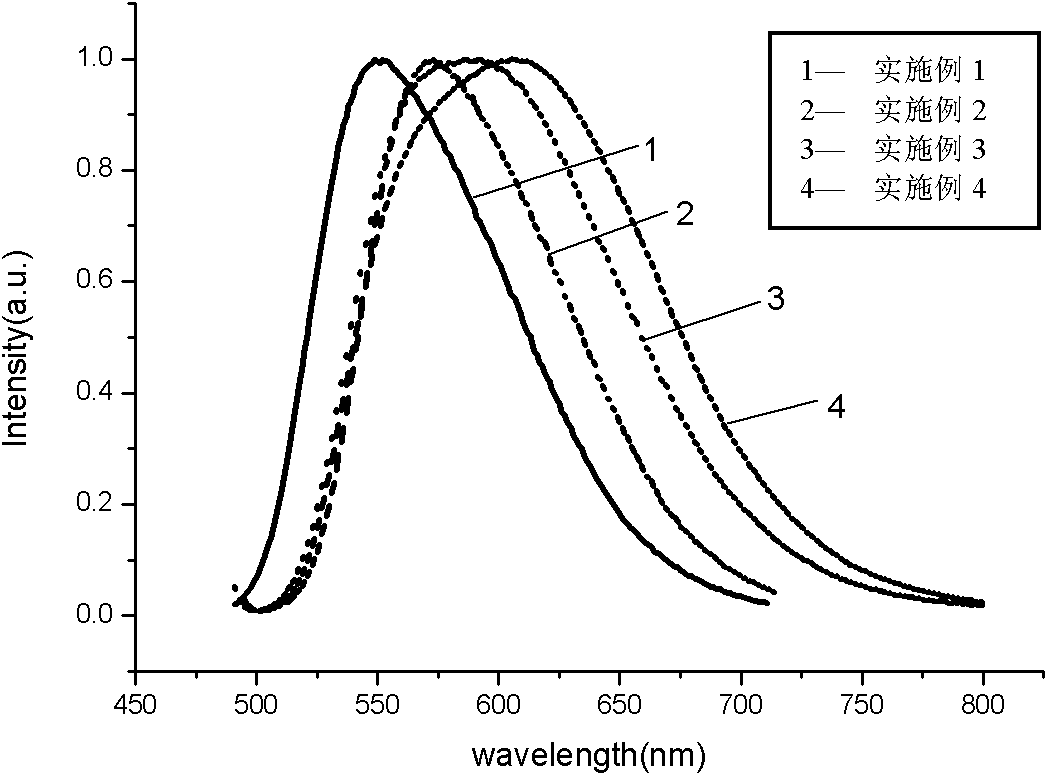
Preparation method of nitrogen oxide yellow fluorescent powder material
A yellow phosphor, nitrogen oxide technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of shortening product life, restricting product development, different attenuation of phosphors, etc., to prolong the service life and widen the process window. , the effect of low price of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The first step to synthesize the precursor Ca of nitrogen oxide yellow phosphor powder 0.3 Sr 1.66 SiO 4 :
[0019] 1) According to the stoichiometric ratio of each element of Ca0.3Sr1.66SiO4, take SrCO310g, CaCO30.123g, SiO20.245g, and account for 3% catalyst SrF2 of the total mass of SrCO3, CaCO3 and SiO2;
[0020] 2) Put the weighed SrCO3, CaCO3, SiO2 and SrF2 into an alumina crucible after mixing and fully grinding, place the crucible in a tube furnace, sinter in air at 1200°C for 5 hours, and then cool to obtain Ca0.3Sr1. 66SiO4.
[0021] The second step is to synthesize the nitrogen oxide yellow phosphor material Ca 0.15 Sr 0.83 Si 2 o 2 N 2 :0.02Eu:
[0022] 1) According to the stoichiometric ratio of each element in Ca0.15Sr0.83Si2O2N2:0.02Eu, grind and sieve the obtained precursor Ca0.3Sr1.66SiO4, weigh 5g, α-Si3N42.811g and Eu2O30.141g, and finally add The 5% SrF of the material gross mass that takes by weighing and BaF Mixing aid, wherein SrF2: BaF2...
Embodiment 2
[0026] The first step to synthesize the precursor Ba of nitrogen oxide yellow phosphor powder 0.2 Sr 1.8 SiO 4 :
[0027] 1) Weigh SrCO30.743g, BaCO35g, SiO21.13g according to Ba0.2Sr1.8SiO4, and account for 3% catalyst SrF2 of the total mass of SrCO3, BaCO3 and SiO23;
[0028] 2) After mixing and fully grinding, put it in an alumina crucible, place the crucible in a tube furnace, sinter in air at 1200°C for 5 hours, and then cool to obtain Ba0.2Sr1.8SiO4.
[0029] The second step is to synthesize the nitrogen oxide yellow phosphor material Ba 0.1 Sr 0.88 Si2 o 2 N 2 :0.02Eu:
[0030] 1) According to the stoichiometric ratio of Ba0.1Sr0.88Si2O2N2:0.02Eu elements, grind and sieve the obtained Ba0.2Sr1.8SiO4, weigh 5g, weigh 2.53g of α-Si3N4 and 0.127g of Eu2O3, and finally add Aforementioned 5% SrF2 and BaF2 of weighing material gross mass are mixed auxiliary agents, wherein SrF2: BaF2=1: 1;
[0031] 2) Put the above-mentioned ingredients into a molybdenum crucible aft...
Embodiment 3
[0035] The first step to synthesize the precursor Ba of nitrogen oxide yellow phosphor powder 1.33 Sr 0.67 SiO 4 :
[0036] 1) Weigh SrCO35.653g, BaCO315g, SiO23.434g according to Ba1.33Sr0.67SiO4, and catalyst SrF2 accounting for 3% of the total mass of the previously weighed substances;
[0037] 2) After mixing and fully grinding, put it in an alumina crucible, place the crucible in a tube furnace, sinter in air at 1200°C for 5 hours, and then cool to obtain Ba1.33Sr0.67SiO4.
[0038] The second step is to synthesize the nitrogen oxide yellow phosphor material Ba 0.635 Sr 0.327 Si 2 o 2 N 2 :0.02Eu:
[0039] 1) After grinding and sieving the obtained Ba1.33Sr0.67SiO4 according to the stoichiometric ratio of each element, weigh 5g, then weigh 2.147g of α-Si3N4 and 0.108g of Eu2O3, and finally add 5% of the total mass of the previously weighed substances The mixing aid of SrF2 and BaF2, wherein SrF2: BaF2=1: 1;
[0040] 2) Put the above-mentioned ingredients into a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com