Formula and preparation process of aztreonam for injection
A preparation process and technology of aztreonam, applied in the field of aztreonam for injection formulation and preparation process thereof, can solve the problem of different clarity, pH and irritation, large difference in the ratio of aztreonam and arginine, high water content, etc. problems, to achieve the effect of shortening the time of removing moisture, good appearance and quality, proportion and content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
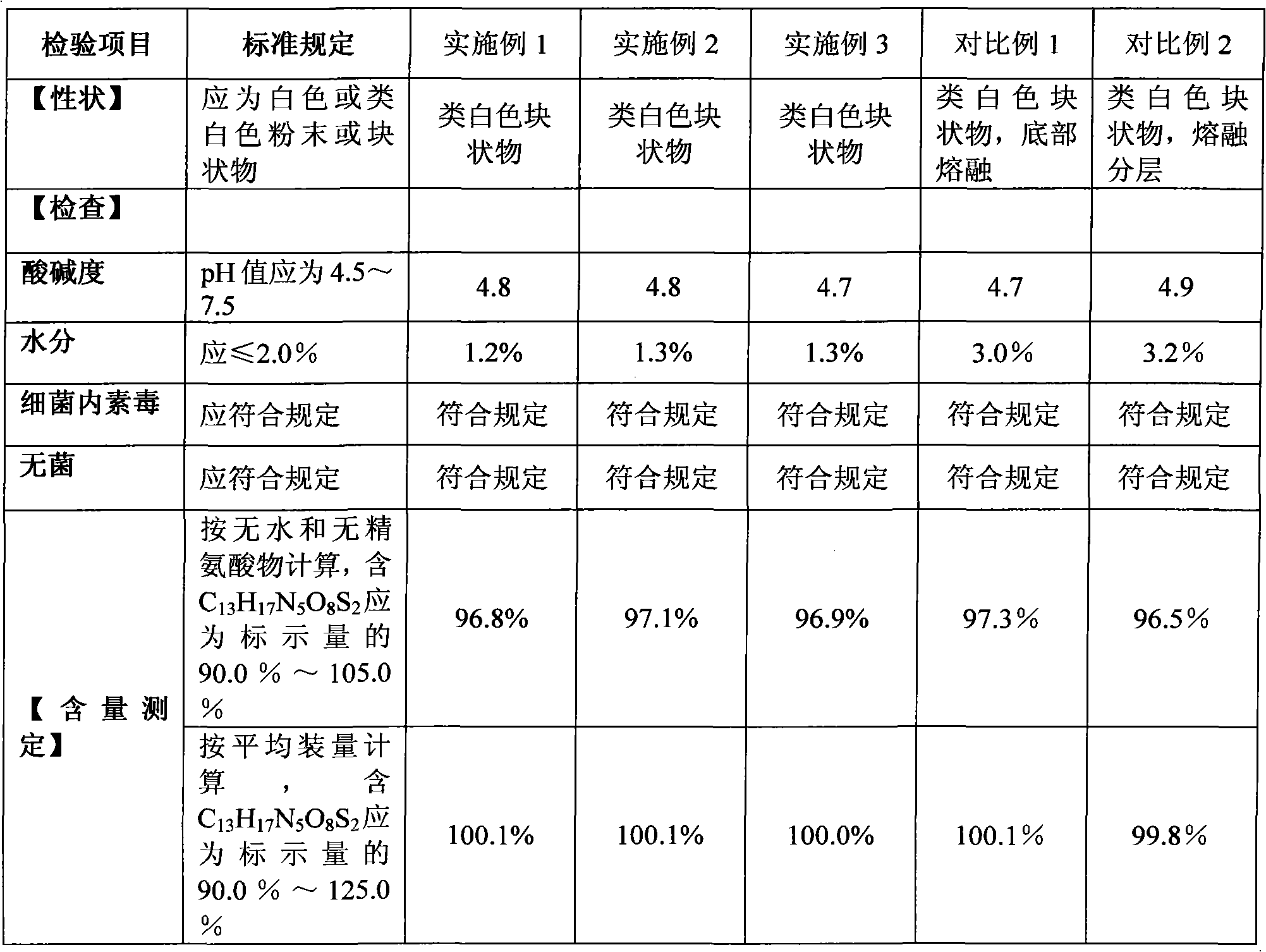
Examples
Embodiment 1
[0037] A kind of prescription of aztreonam for injection is as follows:
[0038] Aztreonam (in C 13 h 17 N 5 o 8 S 2 meter) 500g
[0039] Arginine 400g
[0040] Water for injection 2000g
[0041] A preparation process of aztreonam for injection, the preparation steps are as follows:
[0042] 1. Weighing: first weigh 2000 parts by weight of water for injection and put it in the container, then add 400 parts by weight of arginine to the above container and stir to dissolve, then add 500 parts by weight of aztreonam to the above container Stir to dissolve;
[0043] 2. Pyrogen removal: add 0.1% activated carbon for needles to the above container, stir and absorb for 20 minutes, and filter for decarbonization;
[0044] 3. Sterilization: within 8 hours, filter and sterilize the prepared medicinal solution through a 0.22μm filter membrane;
[0045] 4. Filling: fill the sterilized medicinal solution into vials within 8 hours;
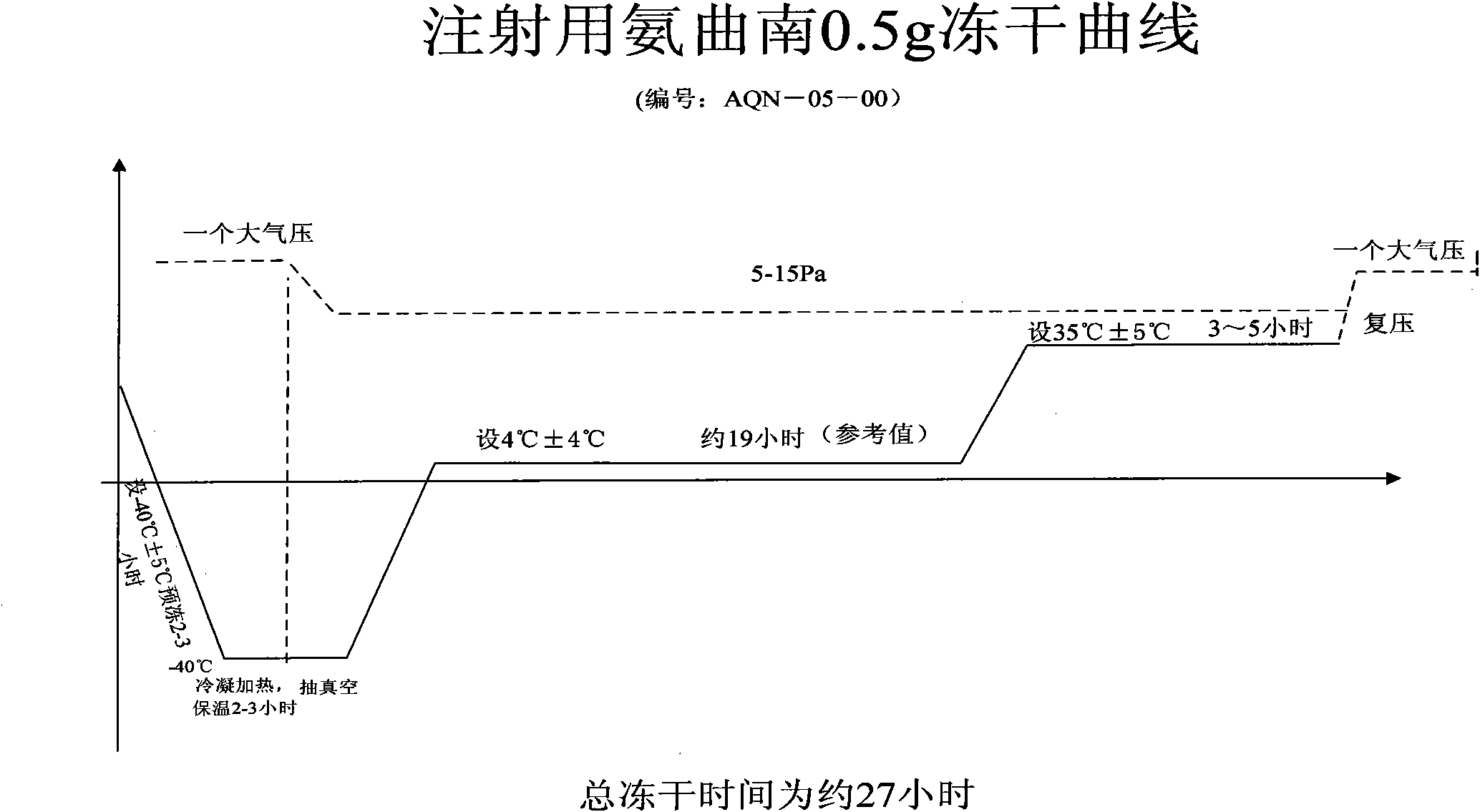
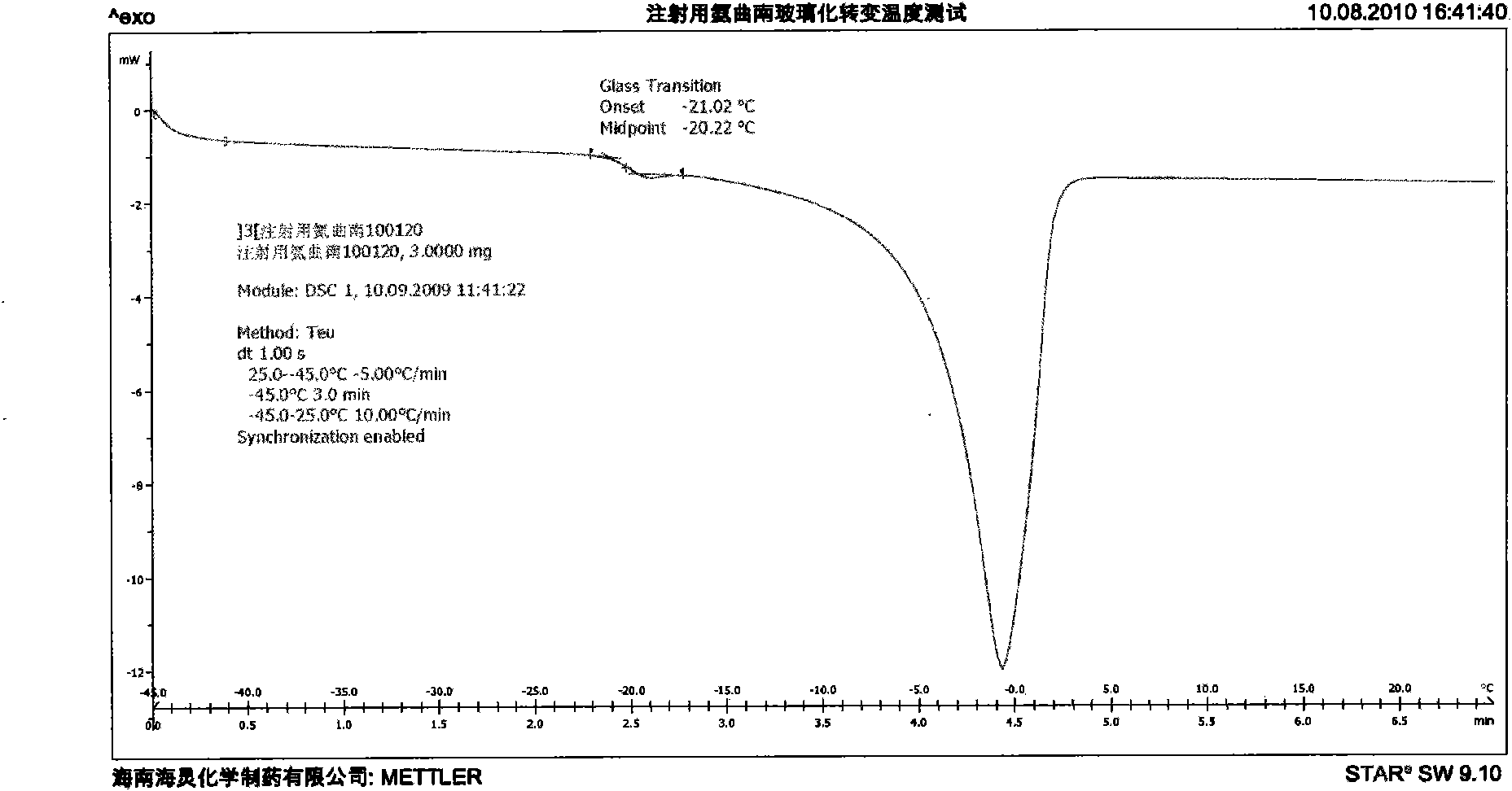
[0046]5. Freeze-drying: transfer the vial cont...
Embodiment 2
[0049] A kind of prescription of aztreonam for injection is as follows:
[0050] Aztreonam (in C 13 h 17 N 5 o 8 S 2 meter) 500g
[0051] Arginine 400g
[0052] Water for injection 2000g
[0053] A preparation process of aztreonam for injection, the preparation steps are as follows:
[0054] 1. Weighing: first weigh 2000 parts by weight of water for injection and put it in the container, then add 400 parts by weight of arginine to the above container and stir to dissolve, then add 500 parts by weight of aztreonam to the above container Stir to dissolve;
[0055] 2. Pyrogen removal: add 0.1% activated carbon for needles to the above container, stir and absorb for 20 minutes, and filter for decarbonization;
[0056] 3. Sterilization: within 8 hours, filter and sterilize the prepared medicinal solution through a 0.22μm filter membrane;
[0057] 4. Filling: fill the sterilized medicinal solution into vials within 8 hours;
[0058] 5. Freeze-drying: Transfer the vial con...
Embodiment 3
[0061] A kind of prescription of aztreonam for injection is as follows:
[0062] Aztreonam (in C 13 h 17 N 5 o 8 S 2 meter) 500g
[0063] Arginine 400g
[0064] Water for injection 2000g
[0065] A preparation process of aztreonam for injection, the preparation steps are as follows:
[0066] 1. Weighing: first weigh 2000 parts by weight of water for injection and put it in the container, then add 400 parts by weight of arginine to the above container and stir to dissolve, then add 500 parts by weight of aztreonam to the above container Stir to dissolve;
[0067] 2. Pyrogen removal: add 0.1% activated carbon for needles to the above container, stir and absorb for 20 minutes, and filter for decarbonization;
[0068] 3. Sterilization: within 8 hours, filter and sterilize the prepared medicinal solution through a 0.22μm filter membrane;
[0069] 4. Filling: fill the sterilized medicinal solution into vials within 8 hours;
[0070] 5. Freeze-drying: transfer the vial con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com