Antithrombin preparation and preparation method thereof
A technology of antithrombin and preparations, which is applied in the field of antithrombin preparations, and can solve the problems of unsuitable patients, poor stability of hirudin, and low transfer rate of active ingredients of medicinal materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
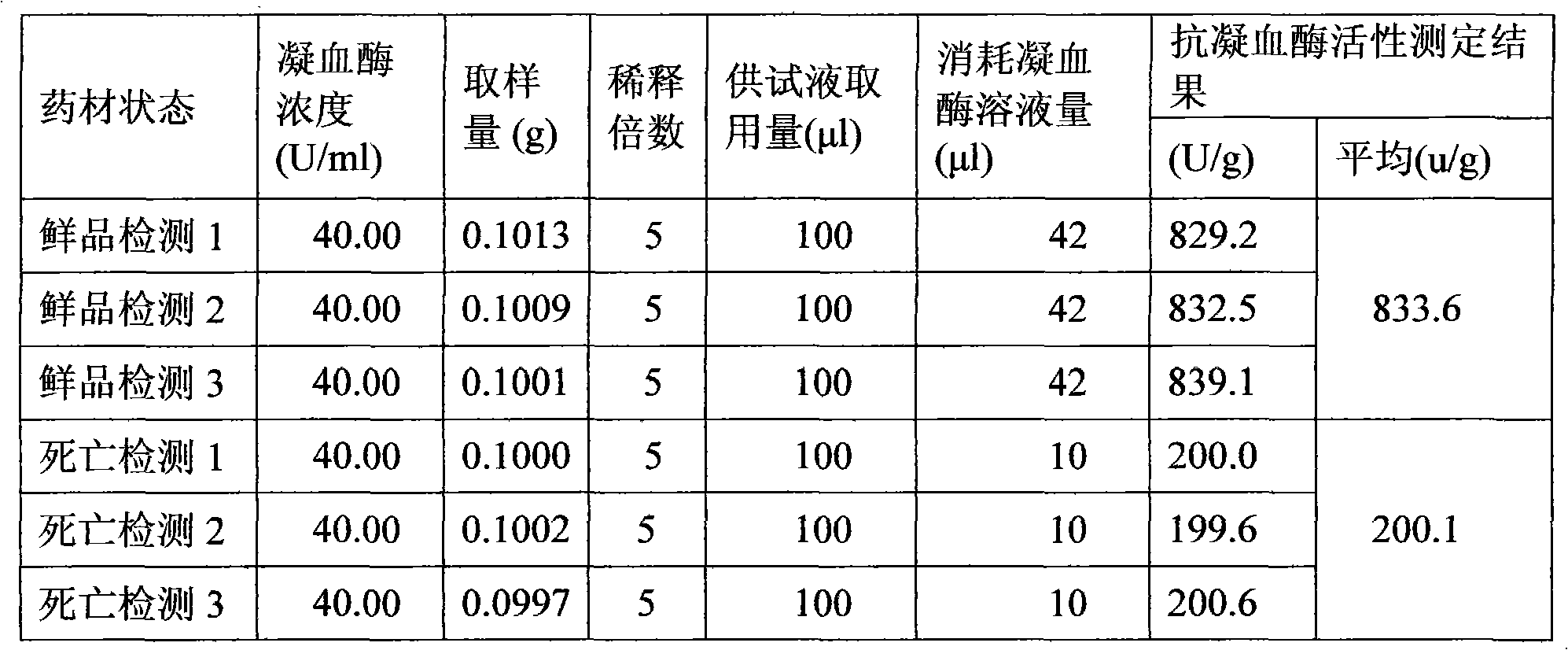
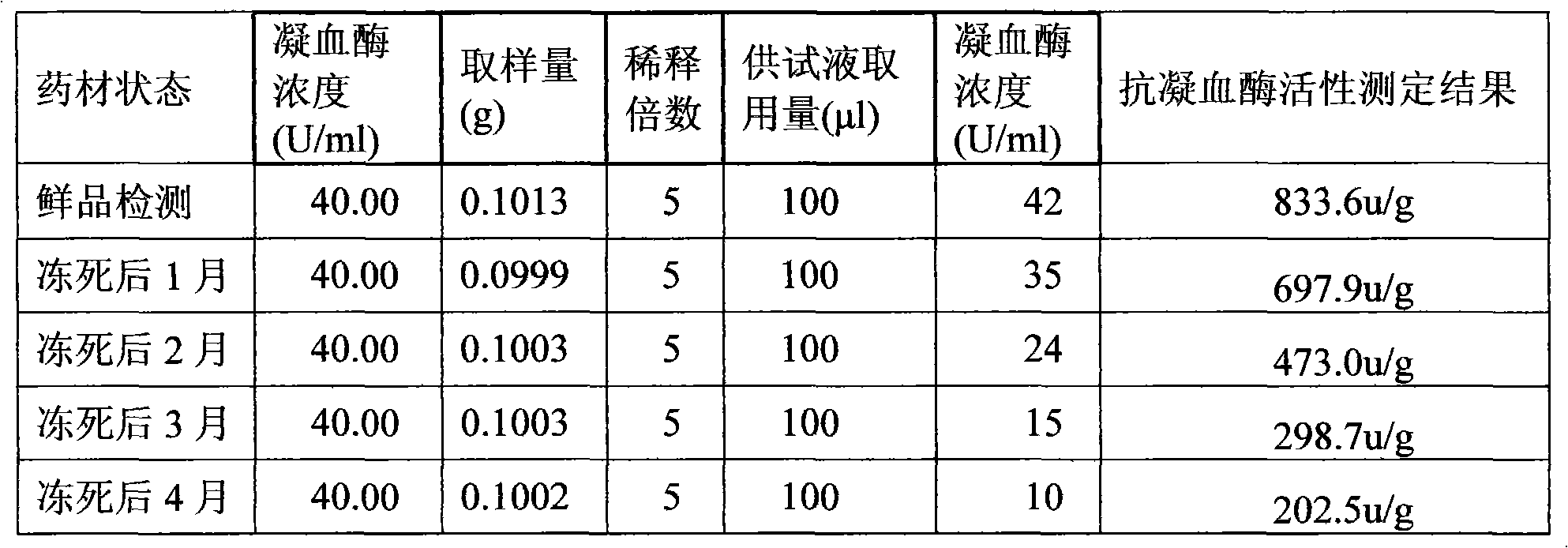
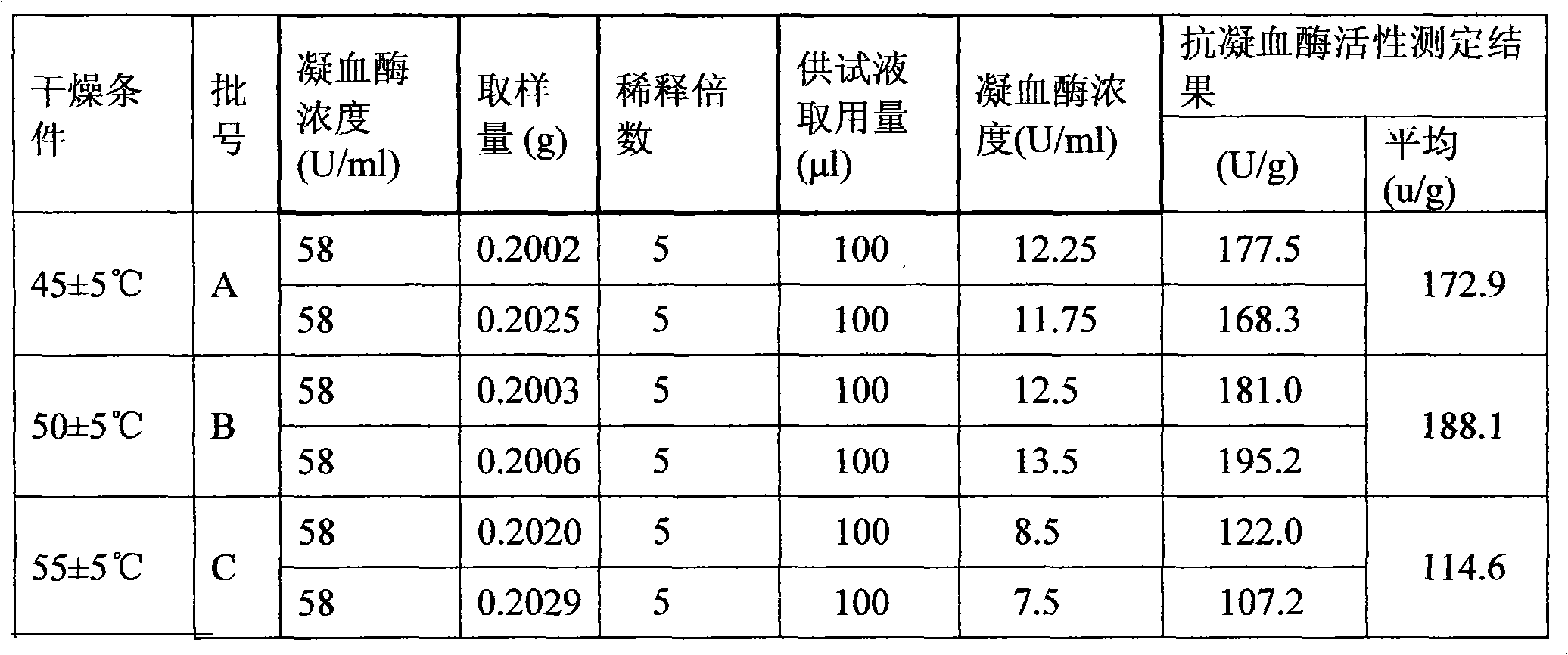
[0120] Preparation of raw drug powder: chop one or more of the frozen leech, leech or phenanthrene into small pieces, process it into a homogenate through a meat grinder, and add the homogenate at a ratio of 1:1.2 After the filler is mixed for 30 minutes, put the mixture in a drying oven, and dry it at a temperature ≤55°C and a vacuum degree ≥-0.09MPa. After drying, grind it to a size above 80 mesh, and then transfer it to a trough mixer for mixing; Adopt the Co60 radiation sterilization method to sterilize the bulk drug powder, and set aside;
[0121] Preparation of soft capsule contents: add weighed beeswax into soybean oil, heat until completely dissolved, and cool to temperature ≤ 45°C; add sterilized raw drug powder, mix well, and pass colloid mill 4 times; Add lecithin and mix evenly, pass through the colloid mill again, and set aside;
[0122] Preparation of enteric-coated soft capsules: Add pure water into the glue tank, turn on the stirring paddle, add glycerin and f...
Embodiment 2
[0124] Preparation of raw drug powder: chop one or more of the frozen leech, leech or phenanthrene into small pieces, process it into a homogenate through a meat grinder, and add the homogenate at a ratio of 1:1.2 After the filler is mixed for 30 minutes, put the mixture in a drying oven, and dry it at a temperature ≤55°C and a vacuum degree ≥-0.09MPa. After drying, grind it to a size above 80 mesh, and then transfer it to a trough mixer for mixing; Adopt the Co60 radiation sterilization method to sterilize the bulk drug powder, and set aside;
[0125] Preparation of soft capsule contents: add weighed beeswax into soybean oil, heat until completely dissolved, and cool to temperature ≤ 45°C; add sterilized raw drug powder, mix well, and pass colloid mill 4 times; Add lecithin and mix evenly, pass through the colloid mill again, and set aside;
[0126] Preparation of enteric-coated soft capsules: Add pure water into the glue tank, turn on the stirring paddle, add glycerin and g...
Embodiment 3
[0128] Preparation of raw drug powder: chop one or more of the frozen leech, leech or phenanthrene into small pieces, process it into a homogenate through a meat grinder, and add the homogenate at a ratio of 1:1.2 After the filler is mixed for 30 minutes, put the mixture in a drying oven, and dry it at a temperature ≤55°C and a vacuum degree ≥-0.09MPa. After drying, grind it to a size above 80 mesh, and then transfer it to a trough mixer for mixing; Adopt the Co60 radiation sterilization method to sterilize the bulk drug powder, and set aside;
[0129] Preparation of soft capsule contents: add weighed beeswax into soybean oil, heat until completely dissolved, and cool to temperature ≤ 45°C; add sterilized raw drug powder, mix well, and pass colloid mill 4 times; Add lecithin and mix evenly, pass through the colloid mill again, and set aside;
[0130] Preparation of enteric-coated soft capsules: Add pure water into the glue tank, turn on the stirring paddle, add glycerin and g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com