Method for preparing nano nickel bicarbonate
A nickel bicarbonate and nanotechnology, applied in the direction of nickel carbonate, can solve few problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
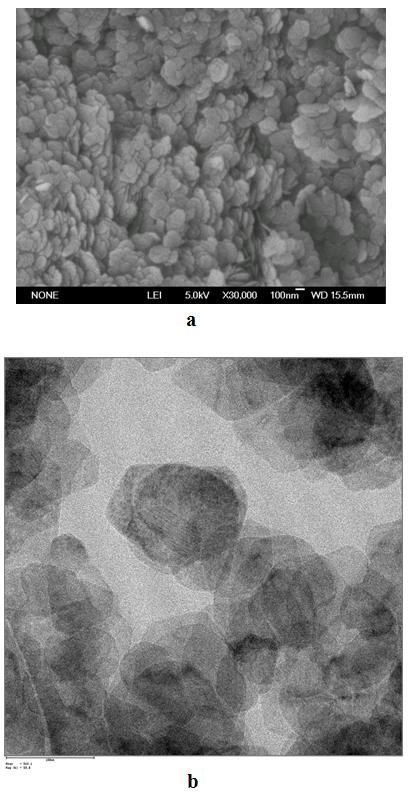
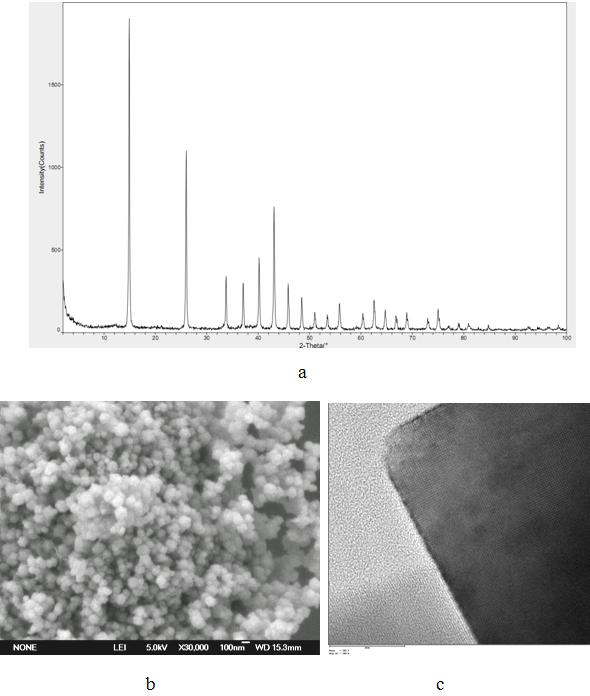
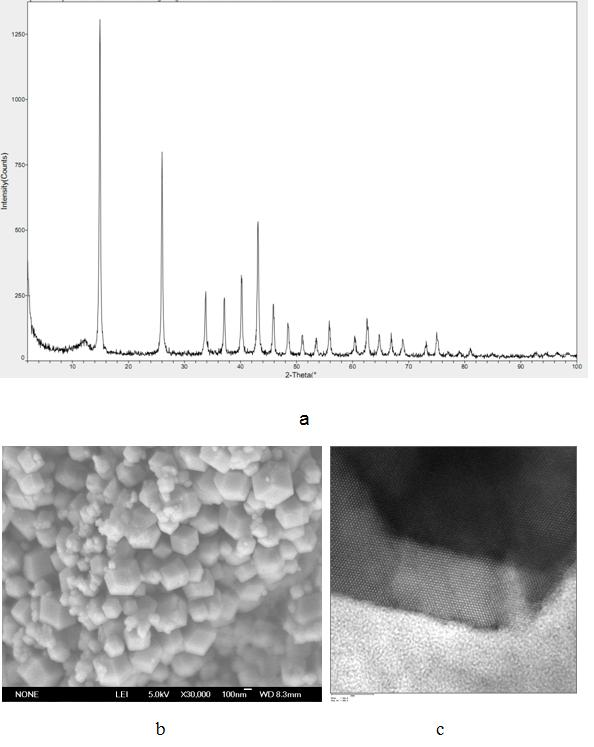
[0022] 1.523 g (0.0064 mol) NiCl 2 ·6H 2 O and 0.341 g (0.0085 mol) NaOH were dissolved in 40 mL distilled water to generate amorphous Ni(OH) 2 Precipitate the suspension, transfer the precipitate to a 50 mL Teflon-lined hydrothermal reaction vessel, and 2 Add 1.536g (0.0256 mol) urea to the precipitate suspension and react at 180°C for 4h. After the hydrothermal reaction was completed, cool down, filter the precipitates of the two samples, wash them with distilled water and absolute ethanol three times, and then dry them in a vacuum oven at 60 °C for 4 h to obtain nano-nickel bicarbonate. Its structural analysis and micrographs are shown in Figure 4 .
Embodiment 2
[0024] 0.995 g (0.004 mol) Ni(CH 3 COO) 2 4H 2 O and 0.192 g (0.0048 mol) NaOH were dissolved in 40 mL of distilled water to generate amorphous Ni(OH) 2 Precipitate the suspension, transfer the precipitate to a 50 mL polytetrafluoroethylene-lined hydrothermal reactor, add 1.922g (0.032mol) urea, and react at 240°C for 1h. After the hydrothermal reaction, cool down, filter the sample precipitate, wash with distilled water and absolute ethanol three times in turn, and then dry in a vacuum oven at 60°C for 4 hours to obtain nano-nickel bicarbonate.
Embodiment 3
[0026] 11.63 g (0.04 mol) Ni(NO 3 ) 2 ·6H 2 O and 4.49 g (0.08 mol) KOH were dissolved in 40 mL distilled water to generate amorphous Ni(OH) 2 Precipitate the suspension, transfer the precipitate to a 50mL polytetrafluoroethylene-lined hydrothermal reactor, add 2.401g (0.04mol) urea, and react at 90°C for 96h. After the hydrothermal reaction, cool down, filter the sample precipitate, wash with distilled water and absolute ethanol three times in turn, and then dry in a vacuum oven at 60°C for 4 hours to obtain nano-nickel bicarbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com