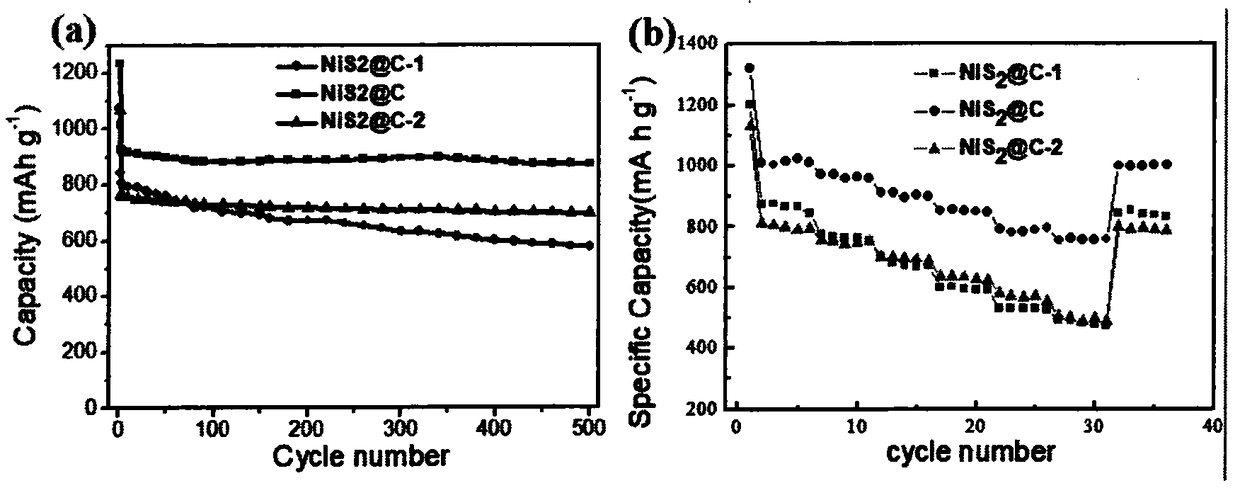
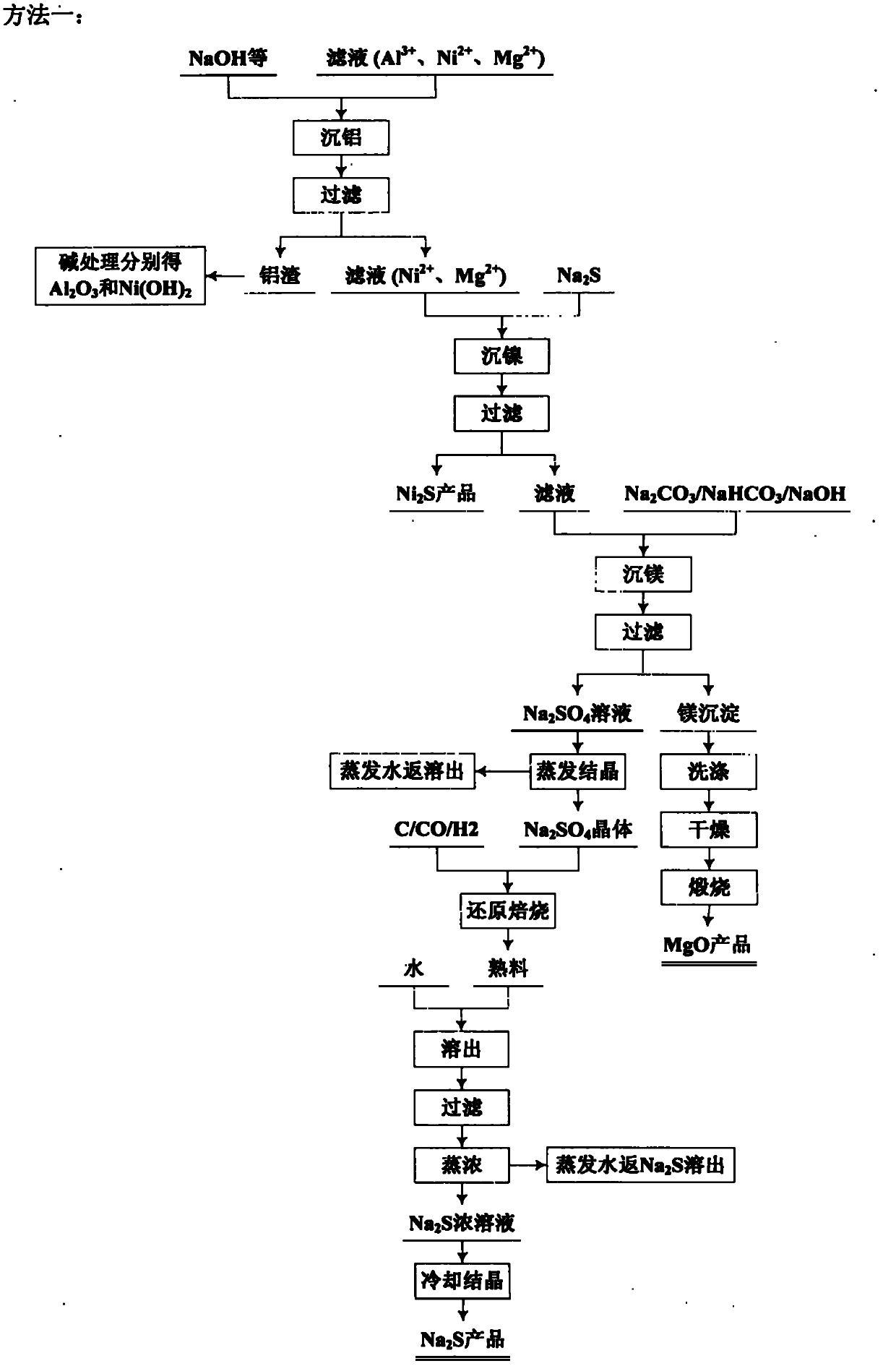
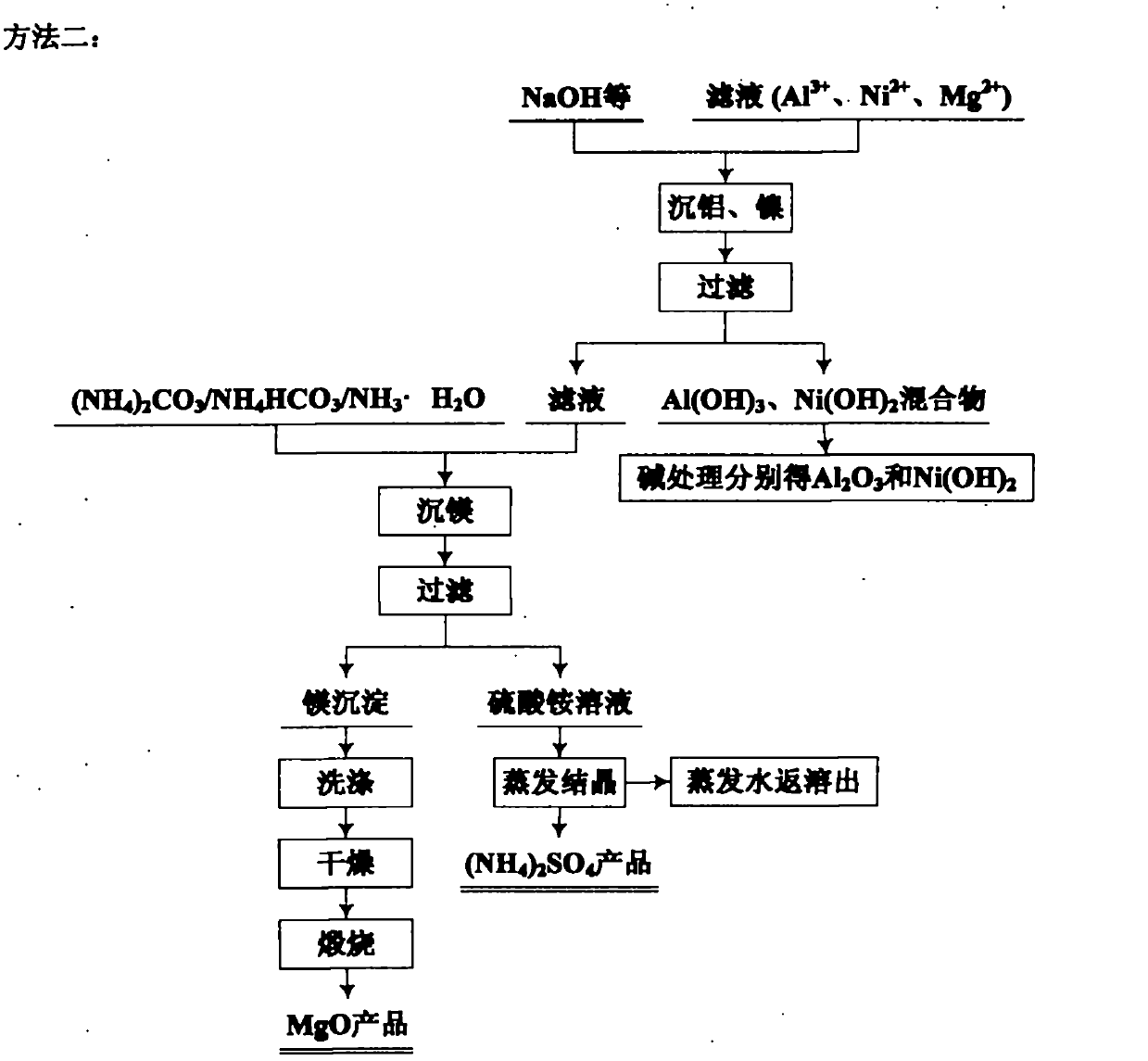
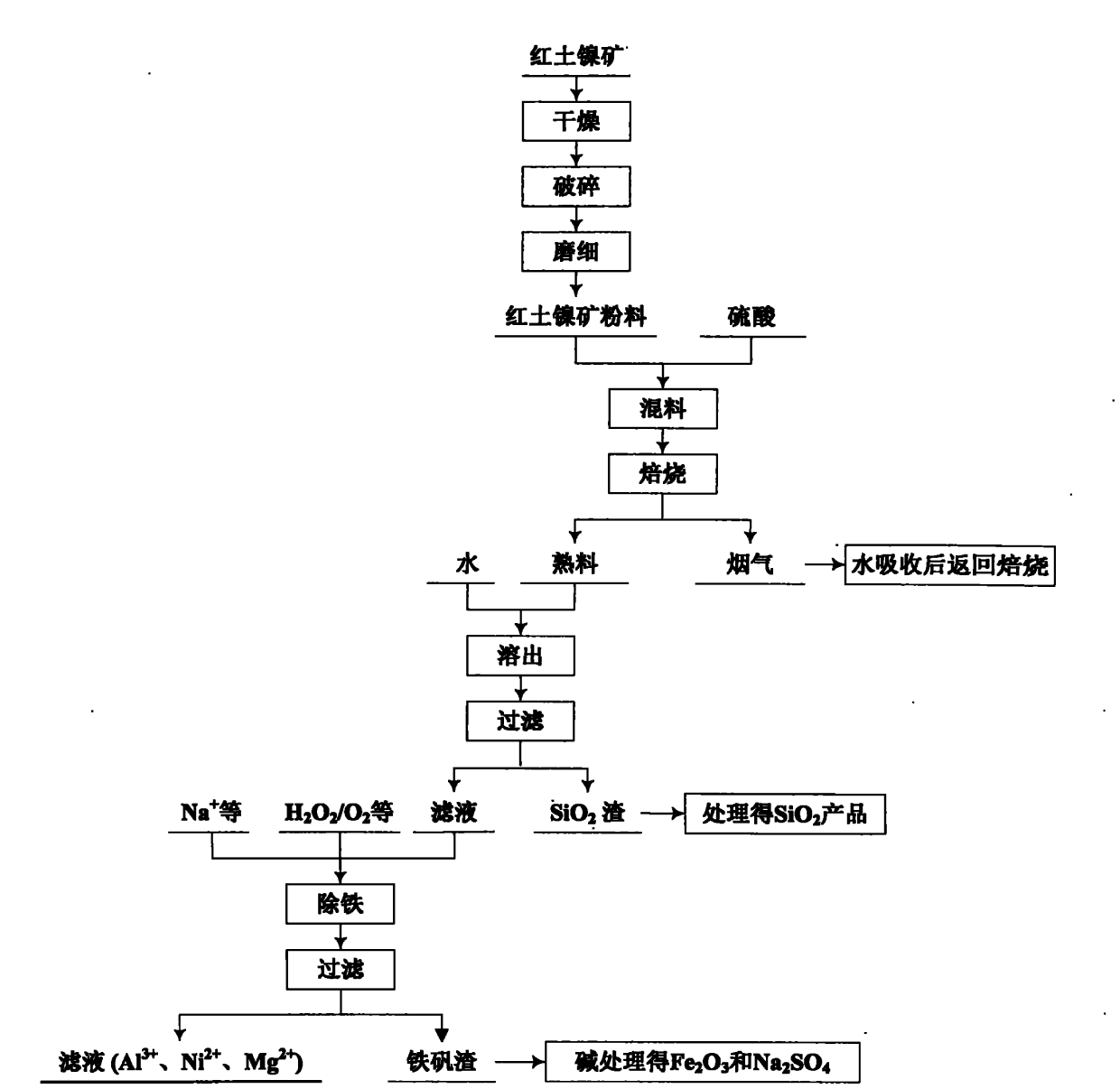
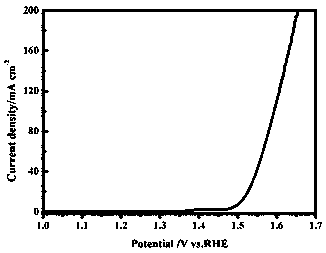
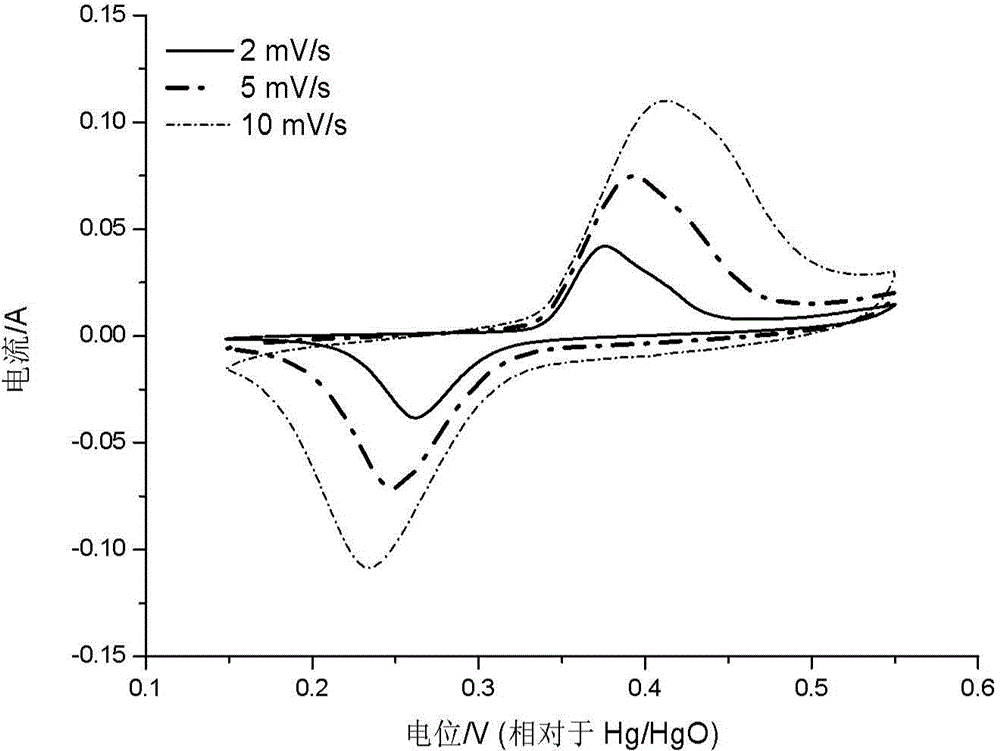
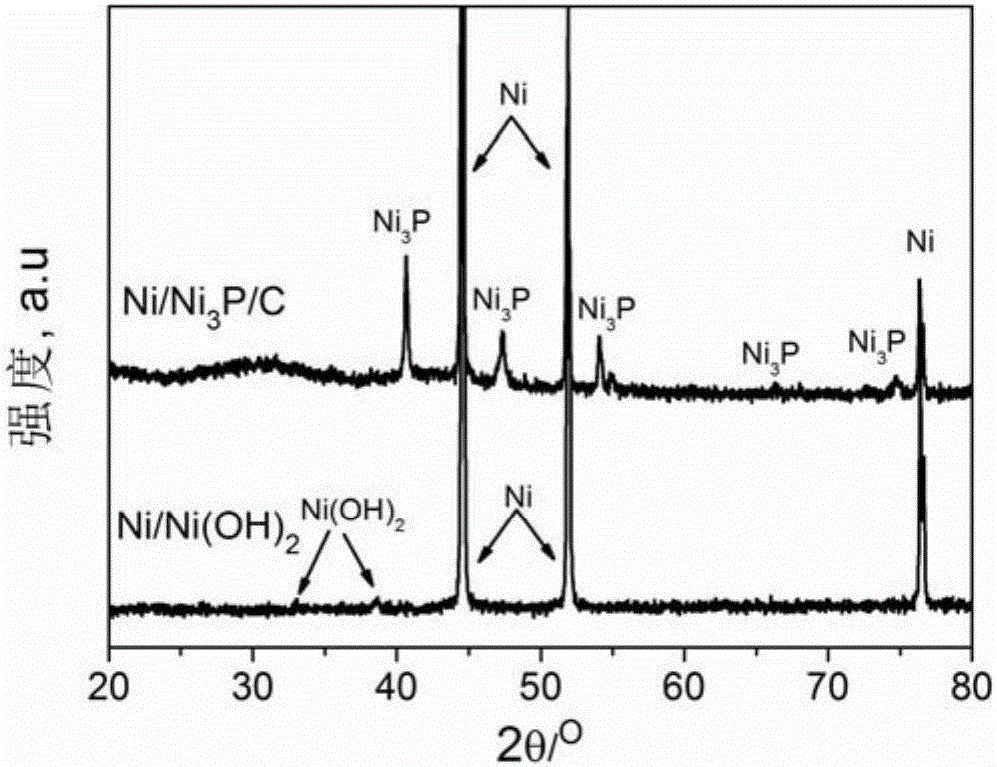
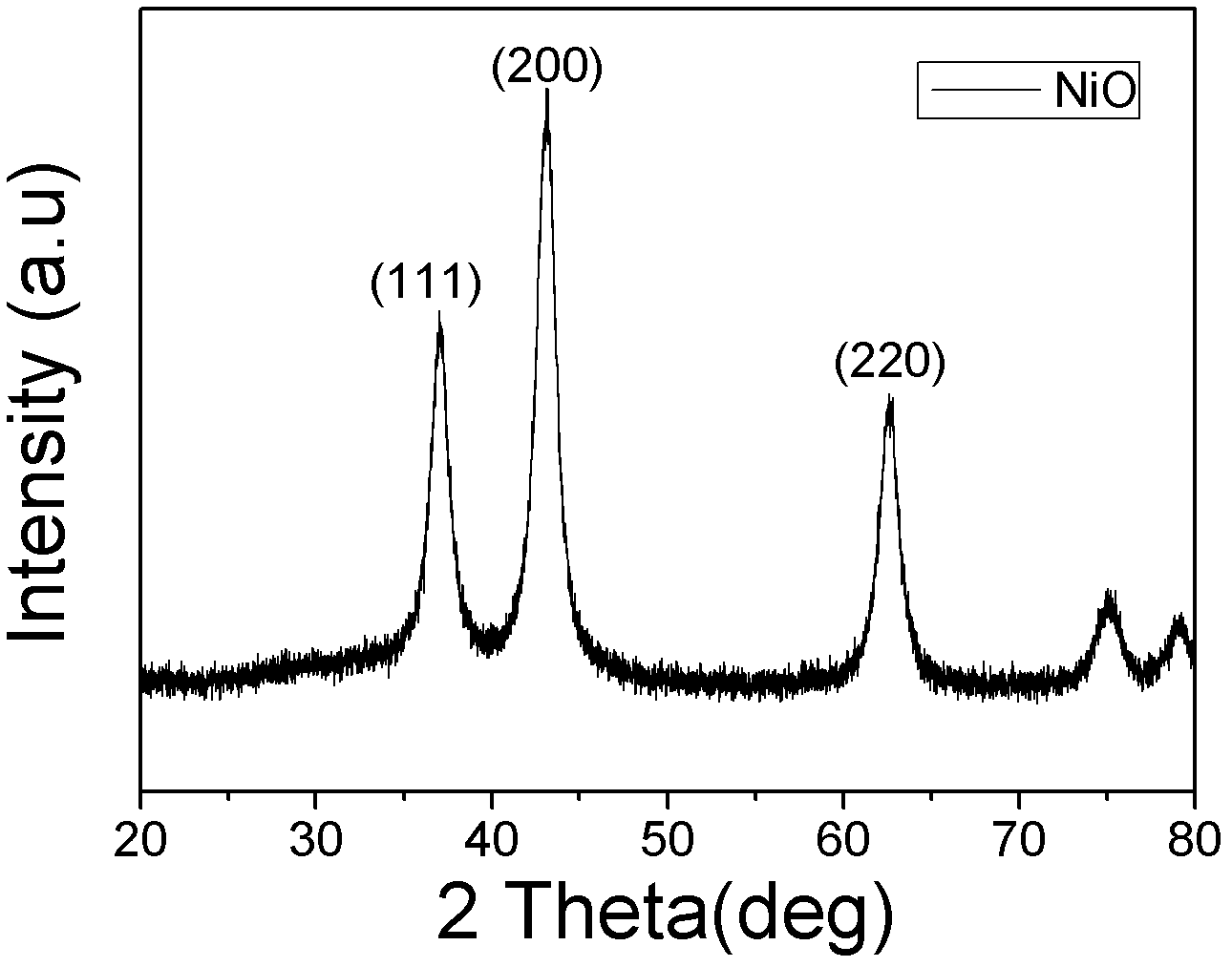
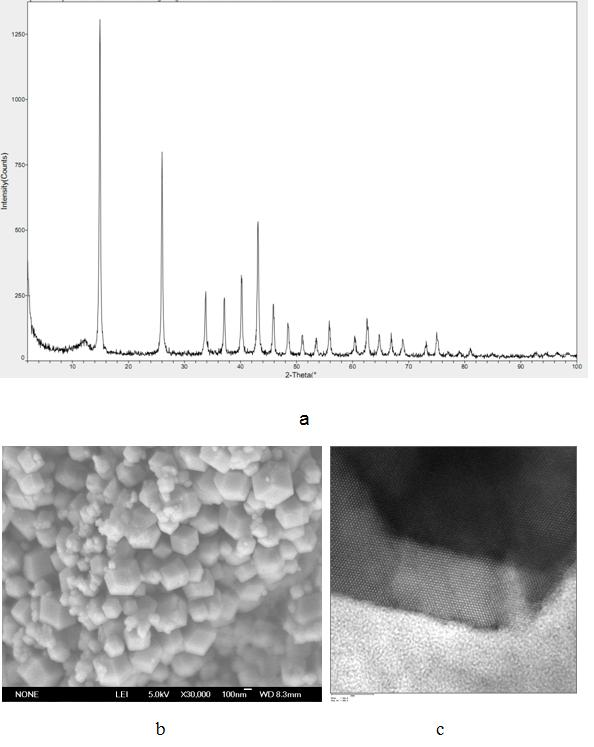
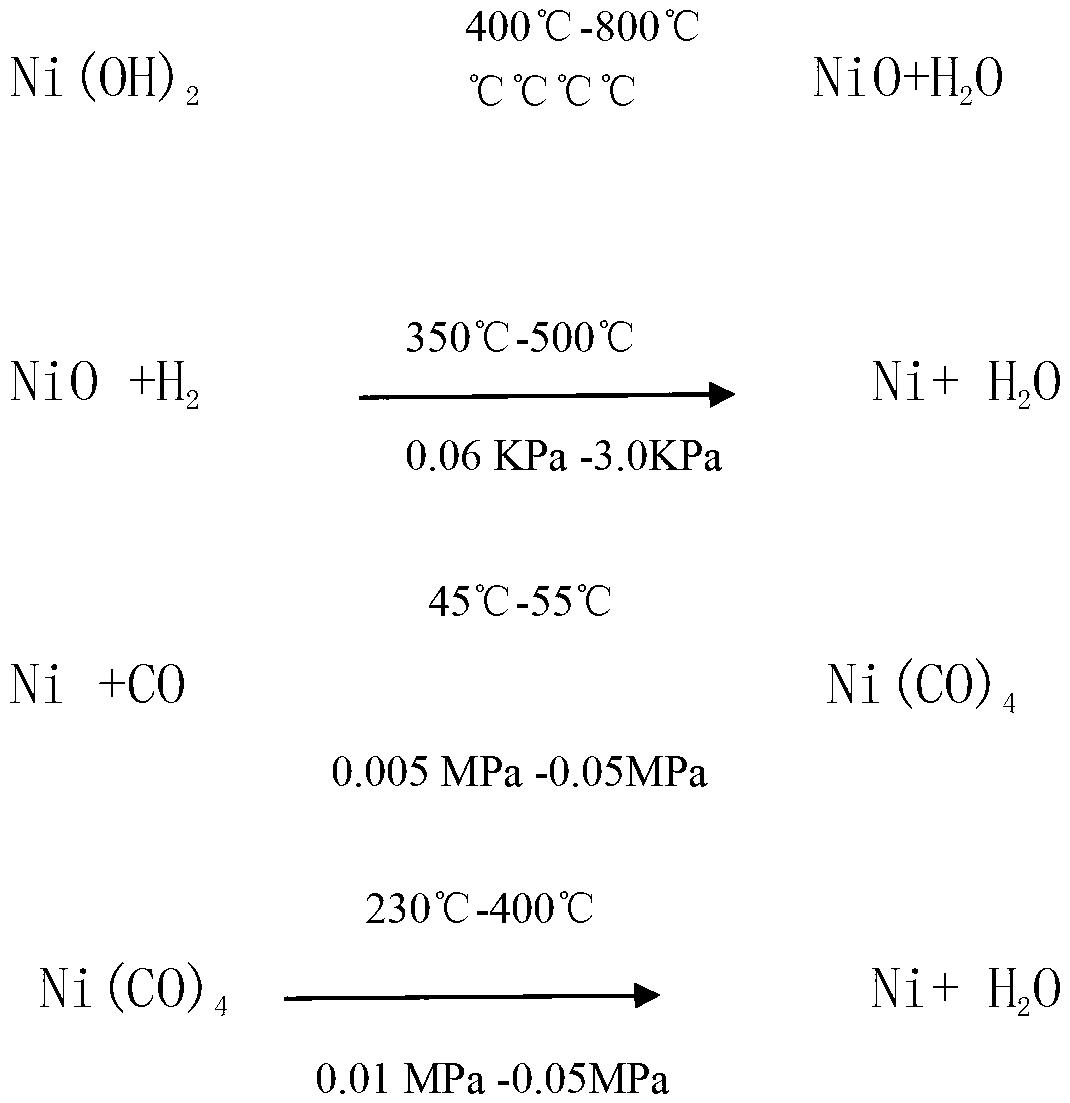
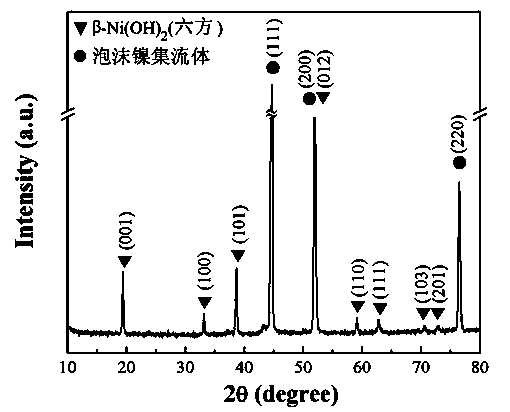
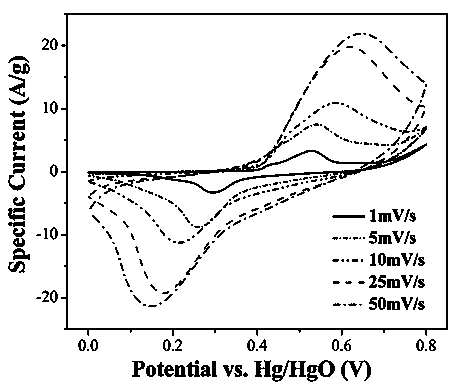
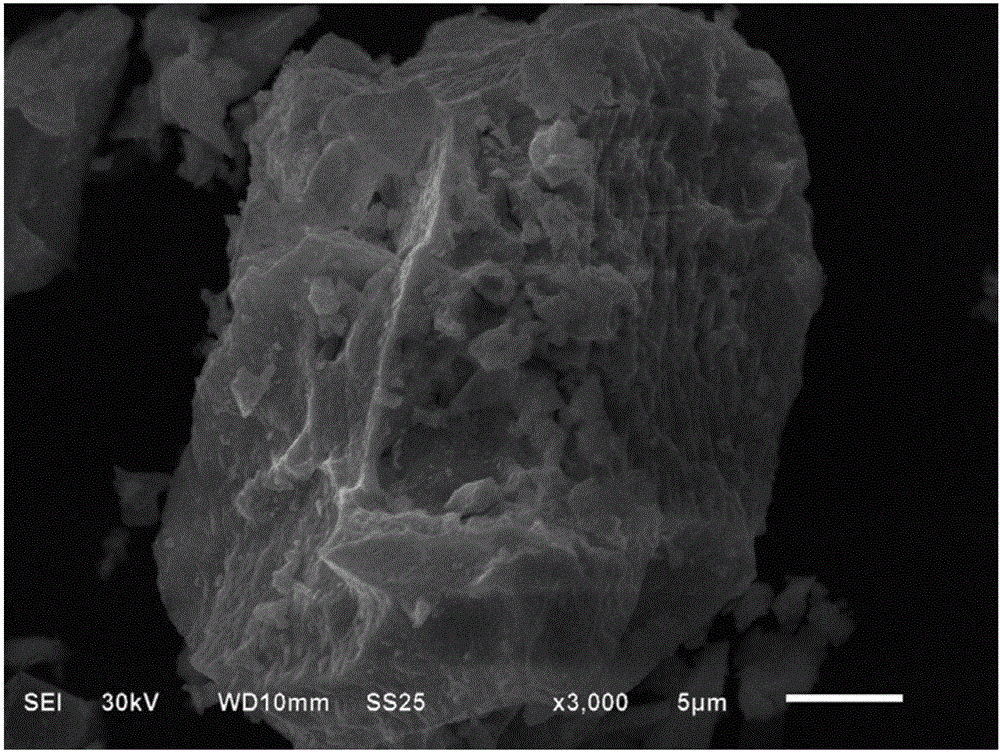
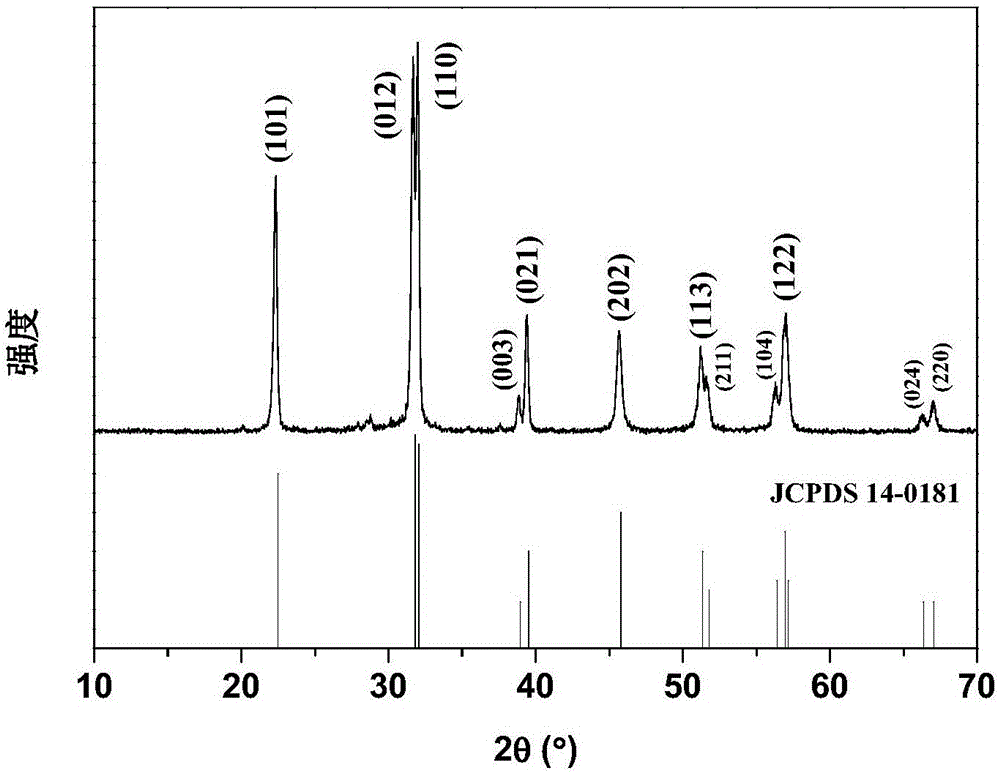
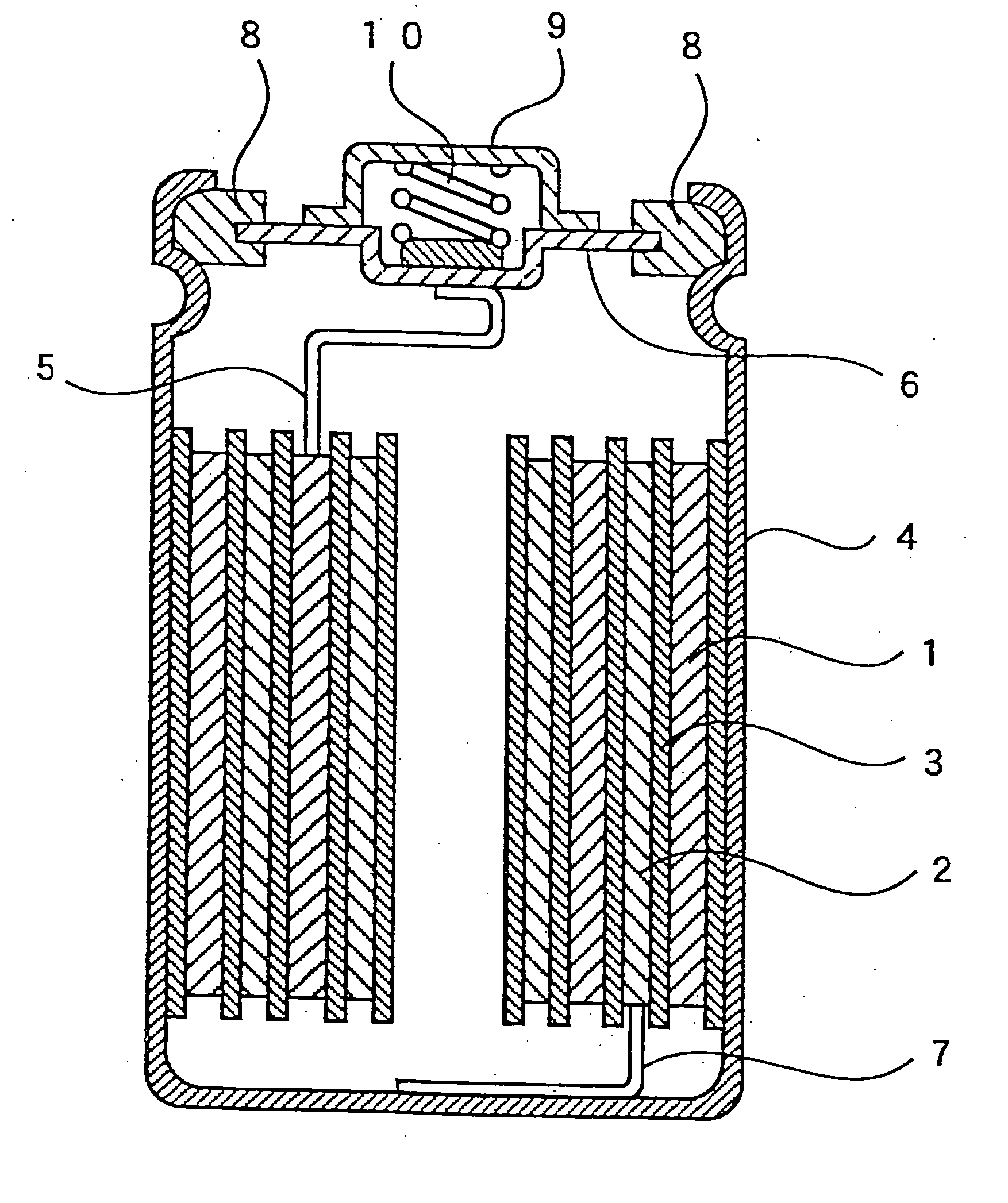
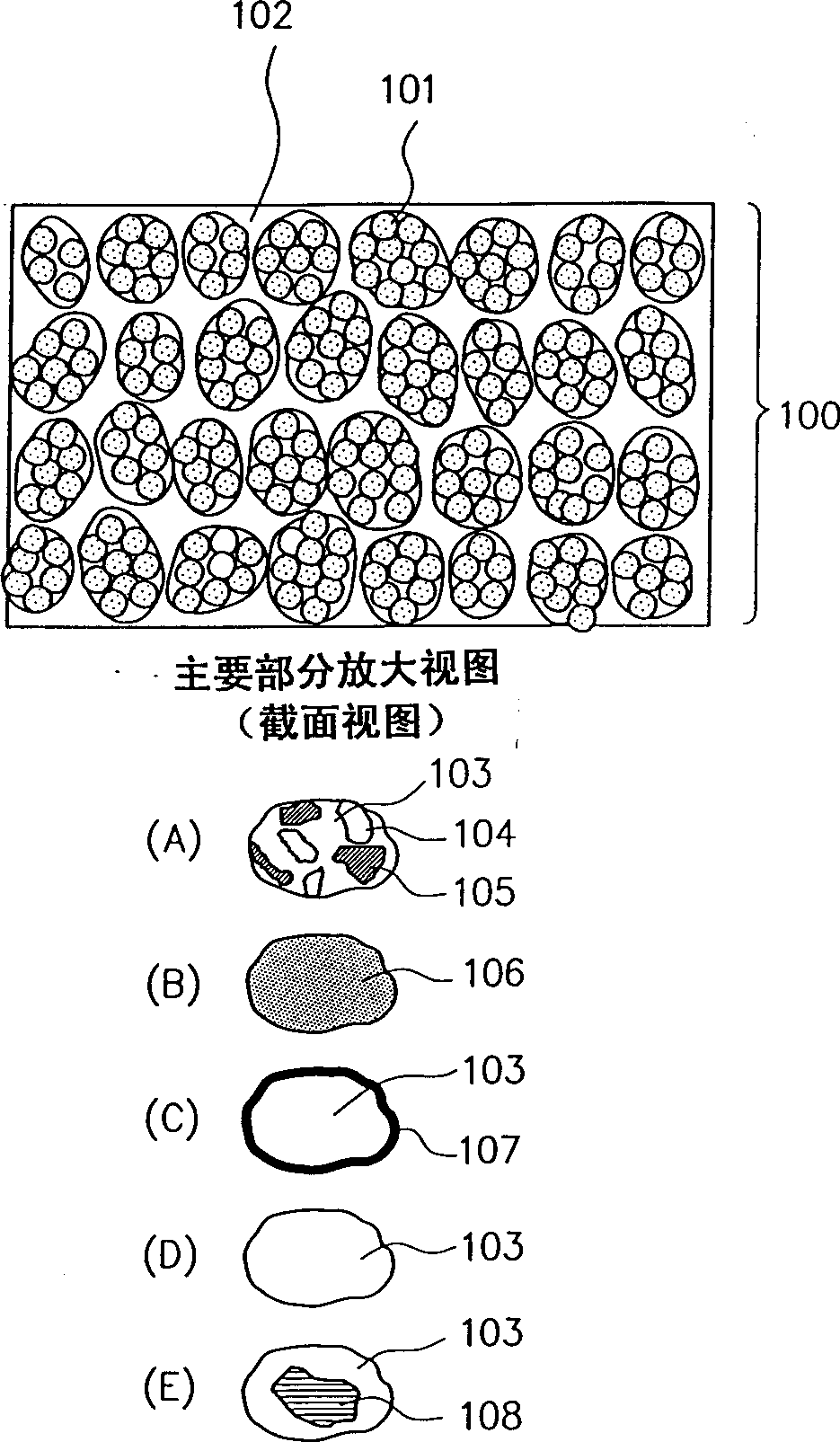
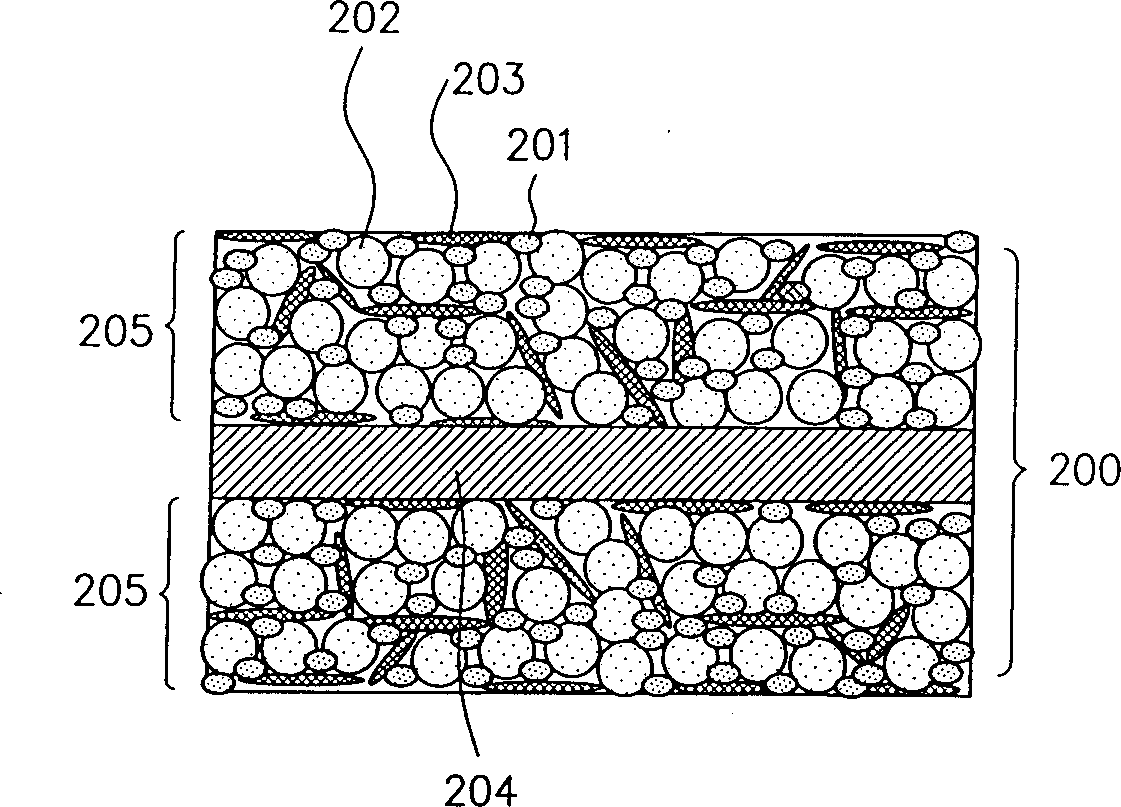
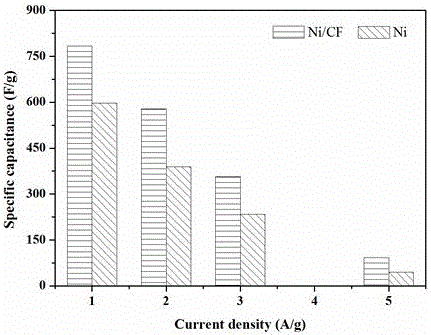
Patents
Literature
377 results about "Nickel hydroxide (II)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for recovering nickel from sulfuric acid aqueous solution
ActiveUS20110135547A1High industrial valueEasy to useSolvent extractionNickel compounds preparationSolventAluminium
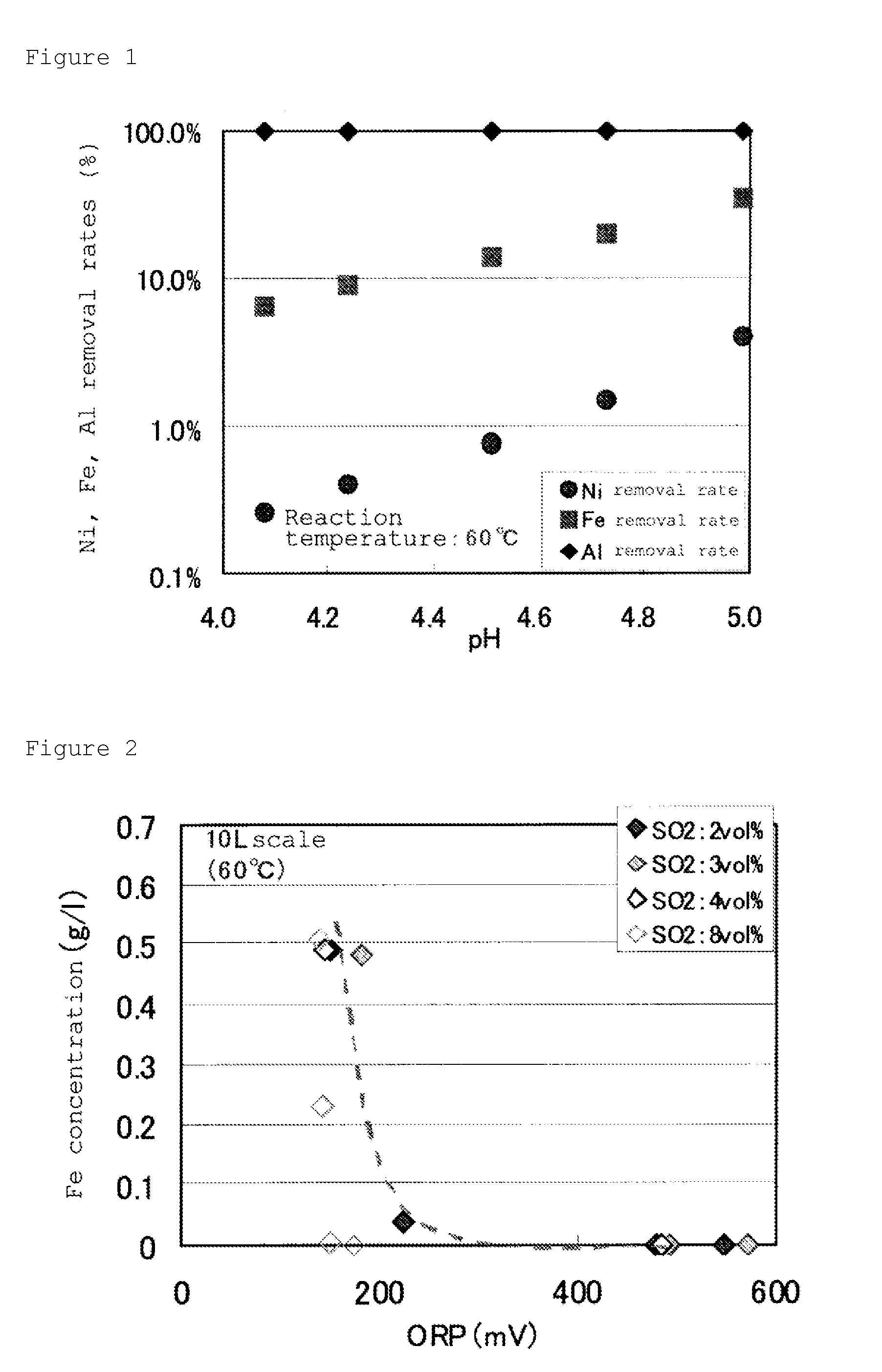
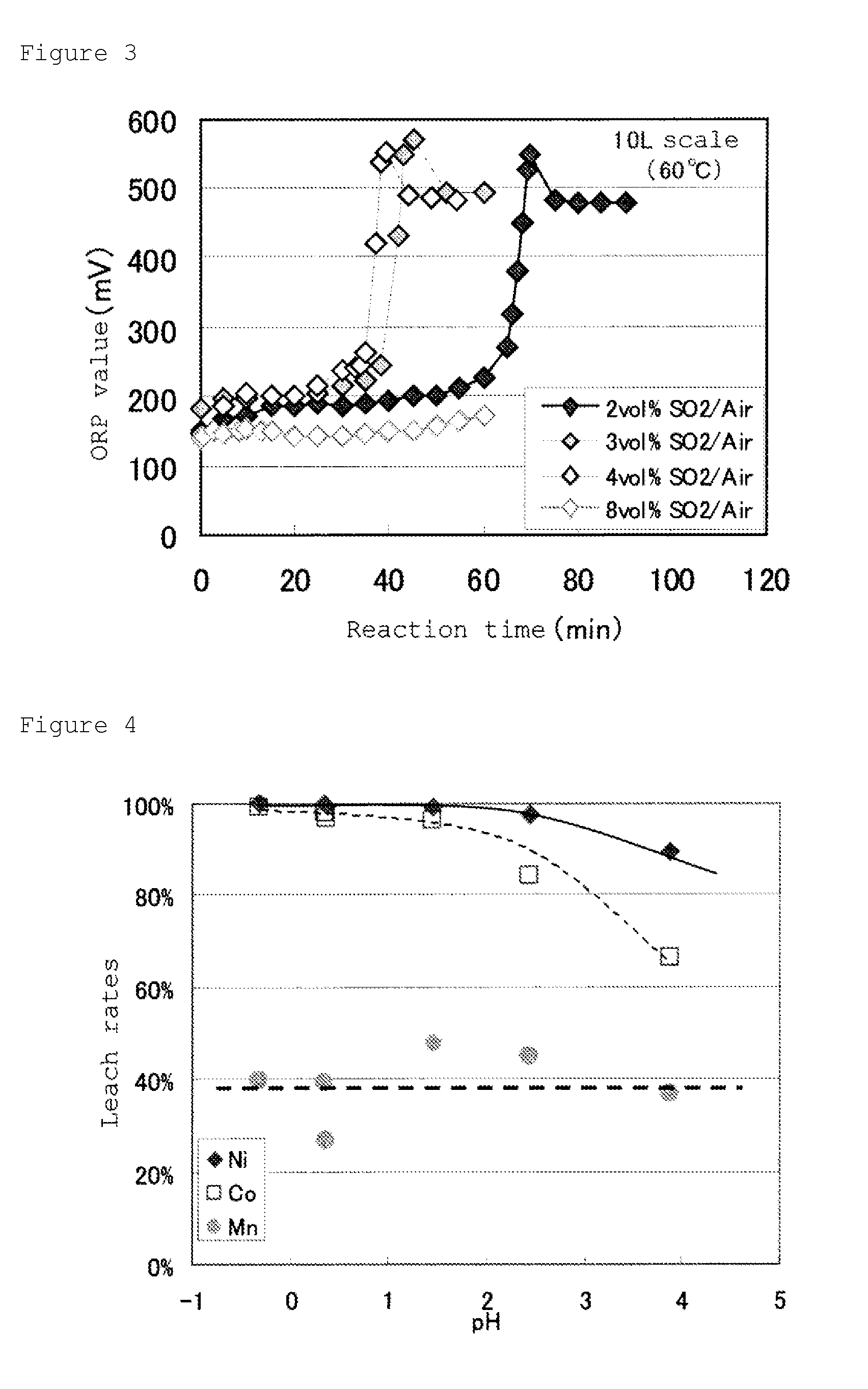
A method for recovering nickel from an sulfuric acid aqueous solution, for recovering nickel in an effectively utilizable form as a raw material of nickel industry material, by separating efficiently impurity elements of iron, aluminum, manganese and the like, from the sulfuric acid aqueous solution containing nickel and cobalt, and the impurity elements, iron, aluminum, manganese and the like.The method is characterized by comprising the following steps (1) to (5).step (1): to subject the sulfuric acid aqueous solution to oxidation neutralization treatment.step (2): then, to subject the solution to neutralization treatment, and to separate and recover mixed hydroxides containing nickel and cobalt.step (3): to subject the mixed hydroxides to dissolution treatment in a sulfuric acid solution having a concentration of equal to or higher than 50% by mass.step (4): to subject the concentrated solution to solvent extraction treatment, using a phosphate ester-based acidic extraction agent.step (5): by adding a neutralizing agent to the resultant extraction residual liquid, to subject the solution to the neutralization treatment, and to separate and recover nickel hydroxide generated.
Owner:SUMITOMO METAL MINING CO LTD
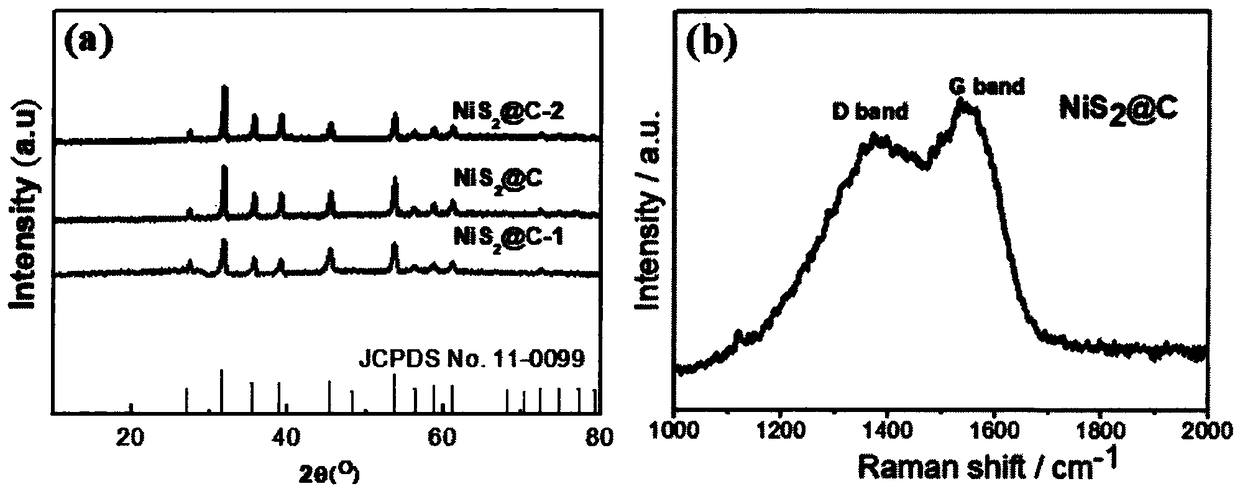
Nickel disulfide carbon nano composite material and preparation method and application thereof
InactiveCN108832097AGood biocompatibilityStrong adhesionMaterial nanotechnologyHybrid capacitor electrodesCarbon compositesCarbon layer
The invention relates to a nickel disulfide carbon nano composite material and a preparation method and an application thereof, wherein the composite material is formed by coating a nickel disulfide nanosheet with a carbon layer. The preparation method comprises the following steps of preparing a nickel hydroxide nanosheet precursor by a hydrothermal method, performing magnetic stirring and dispersing in deionized water to obtain a uniform dispersion liquid of the nickel hydroxide nanosheet precursor, adding a buffering agent tris(hydroxymethyl) aminomethane hydrochloride, and adjusting the pHvalue to be 8.5 by adopting an alkali solution with the pH value of 13, adding dopamine hydrochloride, and magnetically stirring at room temperature for in-situ polymerization, and carrying out washing and centrifugally drying to obtain a nickel hydroxide nanosheet precursor / polydopamine composite material, and carrying out heat treatment and vulcanization with sublimed sulfur powder in a tubularfurnace in nitrogen atmosphere at a certain temperature to obtain the composite material. The preparation process is simple, easy to operate, green and non-toxic and friendly in material preparationprocess; and the prepared nickel disulfide carbon nano composite material is stable in structure, uniform in morphology and high in dispersion. The obtained nickel disulfide carbon nano composite material can be an ideal electrode material of a high-performance lithium ion battery, a supercapacitor and other new energy devices.
Owner:DONGHUA UNIV
Comprehensive utilization method for laterite-nickel ore
The invention relates to an environmental-friendly comprehensive utilization method for a laterite-nickel ore, which comprises the following steps of: (1) grinding the laterite-nickel ore, mixing with sulfuric acid, roasting, dissolving out roasted clinker and filtering to obtain silicon dioxide and dissolution liquid; (2) deironing the dissolution liquid to obtain liquid No.2 and filter residue (iron compounds), wherein the liquid No.2 comprises aluminum, nickel and magnesium and can be treated by the step (3) or (4); (3) precipitating the aluminum in the liquid No.2 by using alkali, filtering, precipitating the nickel in filtrate by using sodium sulfide, filtering, precipitating the magnesium by using the alkali, and treating filter residue to obtain aluminum oxide, nickel hydroxide, nickel sulfide and magnesium oxide respectively; and (4) precipitating the aluminum and the nickel in the liquid No.2 by using the alkali, treating mixed slag containing the aluminum and the nickel by using the alkali to obtain aluminum hydroxide and nickel hydroxide products, and precipitating the magnesium in filtrate subjected to aluminum and nickel precipitation by using ammonia or ammonium saltto obtain a magnesium oxide product. The method is suitable for treating various laterite-nickel ores, three wastes (waste gas, waste water and waste residue) are not generated, and valuable components magnesium, nickel, iron, aluminum and silicon in the laterite-nickel ore are separated and extracted.
Owner:NORTHEASTERN UNIV
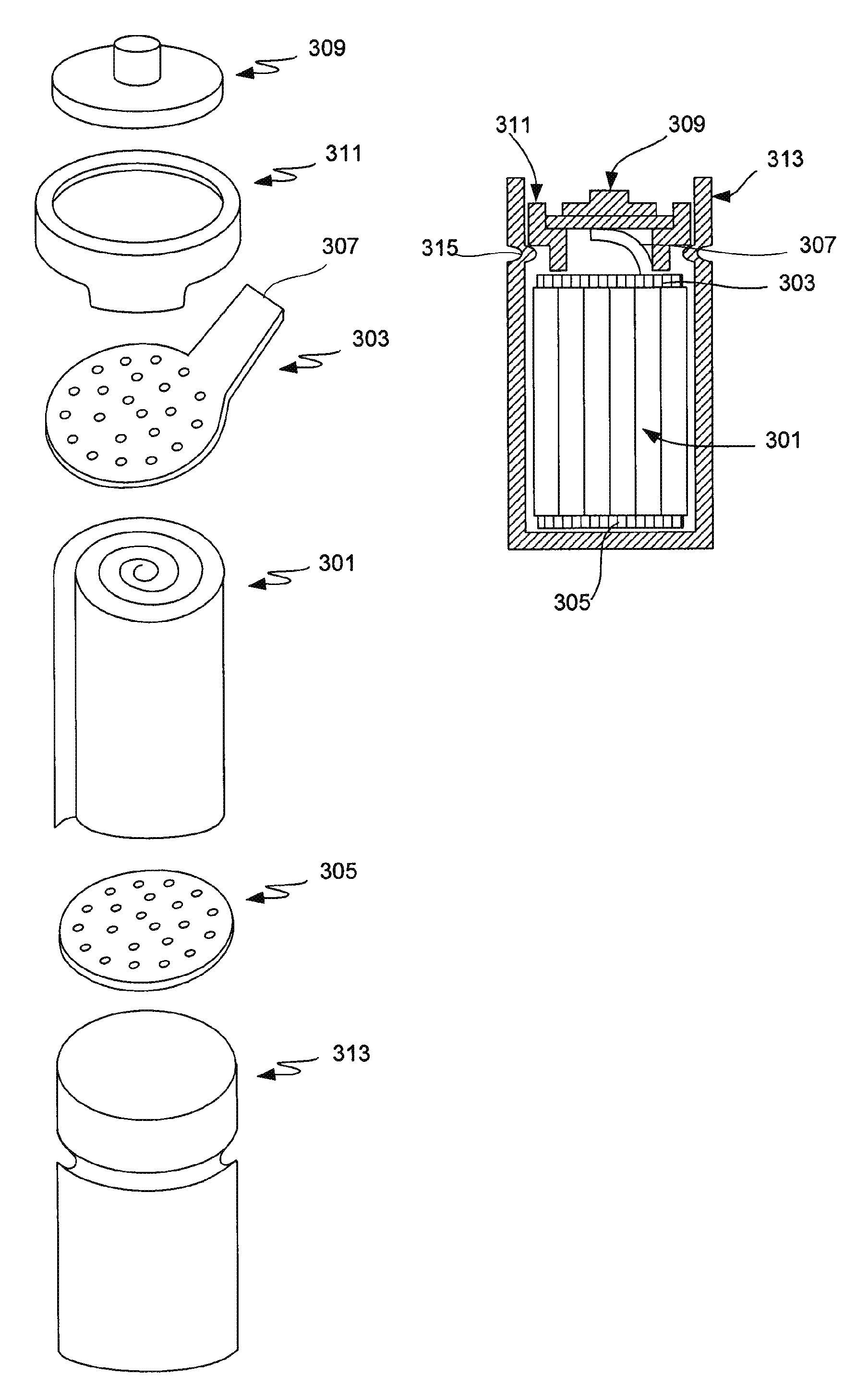
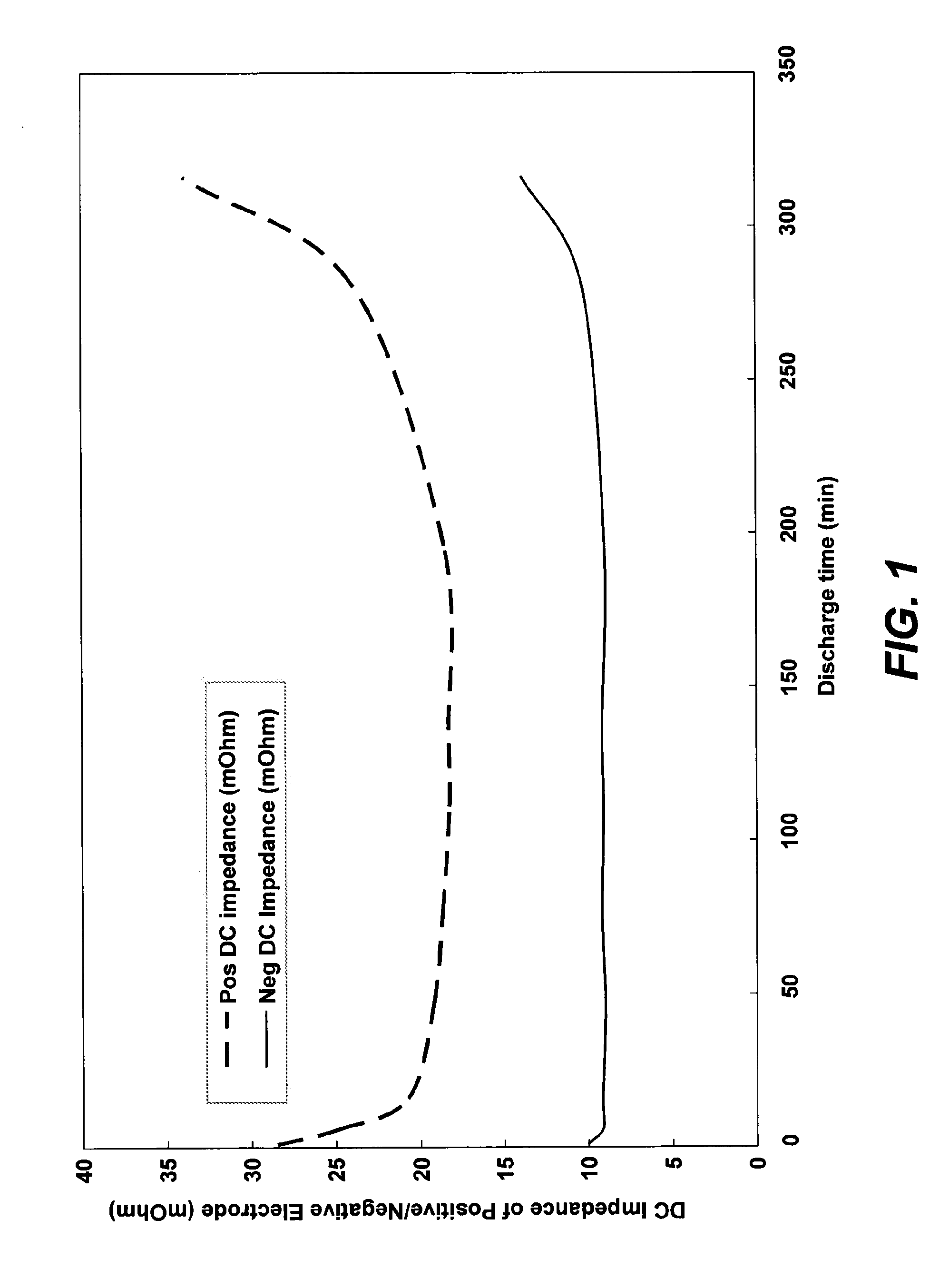
Nickel hydroxide electrode for rechargeable batteries
ActiveUS20090208839A1Facilitate interface reactionImprove reliabilityMaterial nanotechnologyHybrid capacitor electrodesPotassium persulfateNickel oxide hydroxide
The nickel hydroxide particles for a nickel hydroxide electrode may be treated using an alkaline solution of a strong oxidizing agent such as sodium or potassium persulfate to modify the surface nickel hydroxide structure. The resulting modified surface structure has been found to impart various benefits to electrodes formed from the nickel hydroxide. It is believed that the oxidation of cobalt compounds at the surface of the nickel hydroxide particles results in a highly conductive cobalt compound that plays an important role in the high reliability, high stability and high capacity utilization of nickel electrodes as described herein.
Owner:ZINCFIVE POWER INC
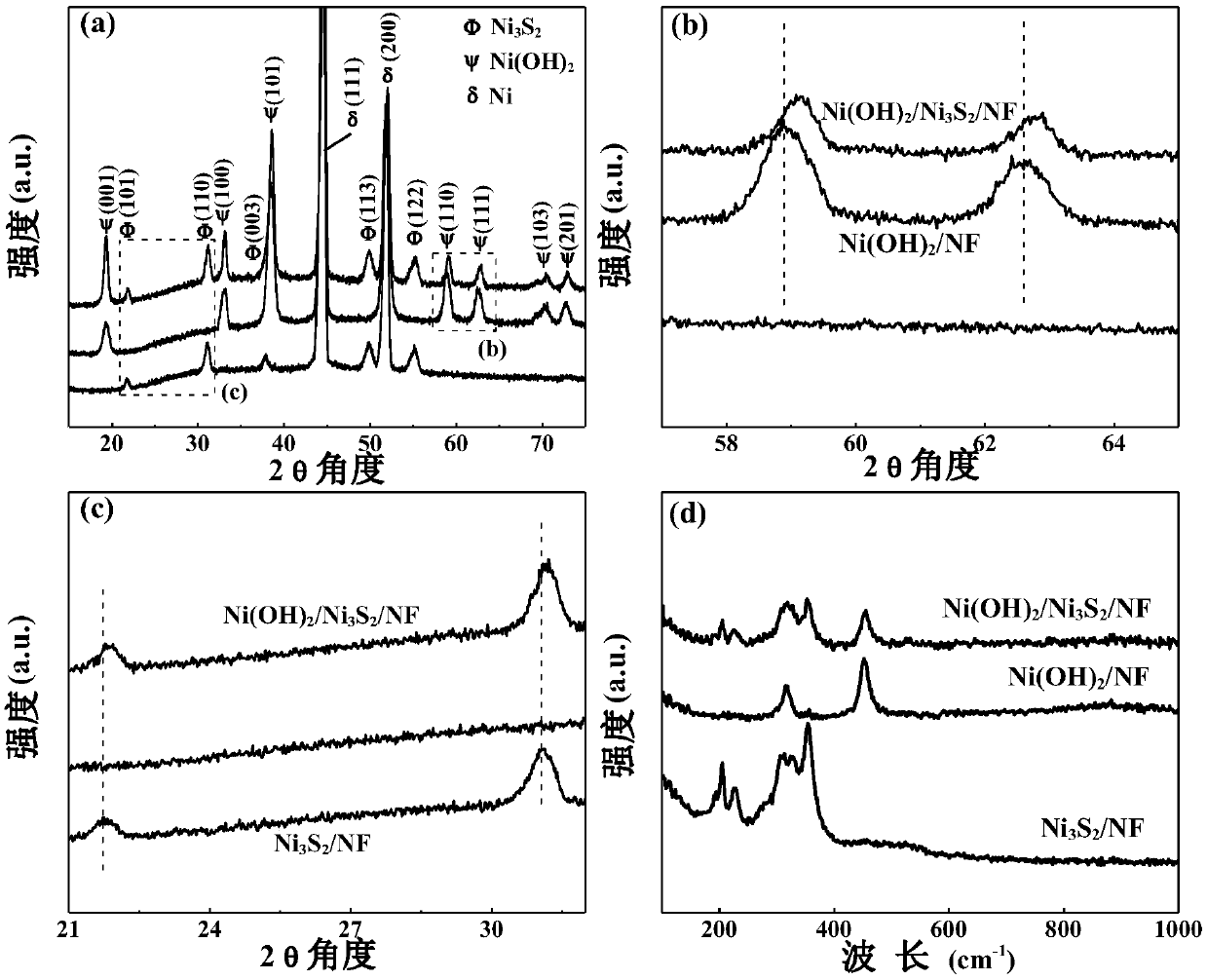
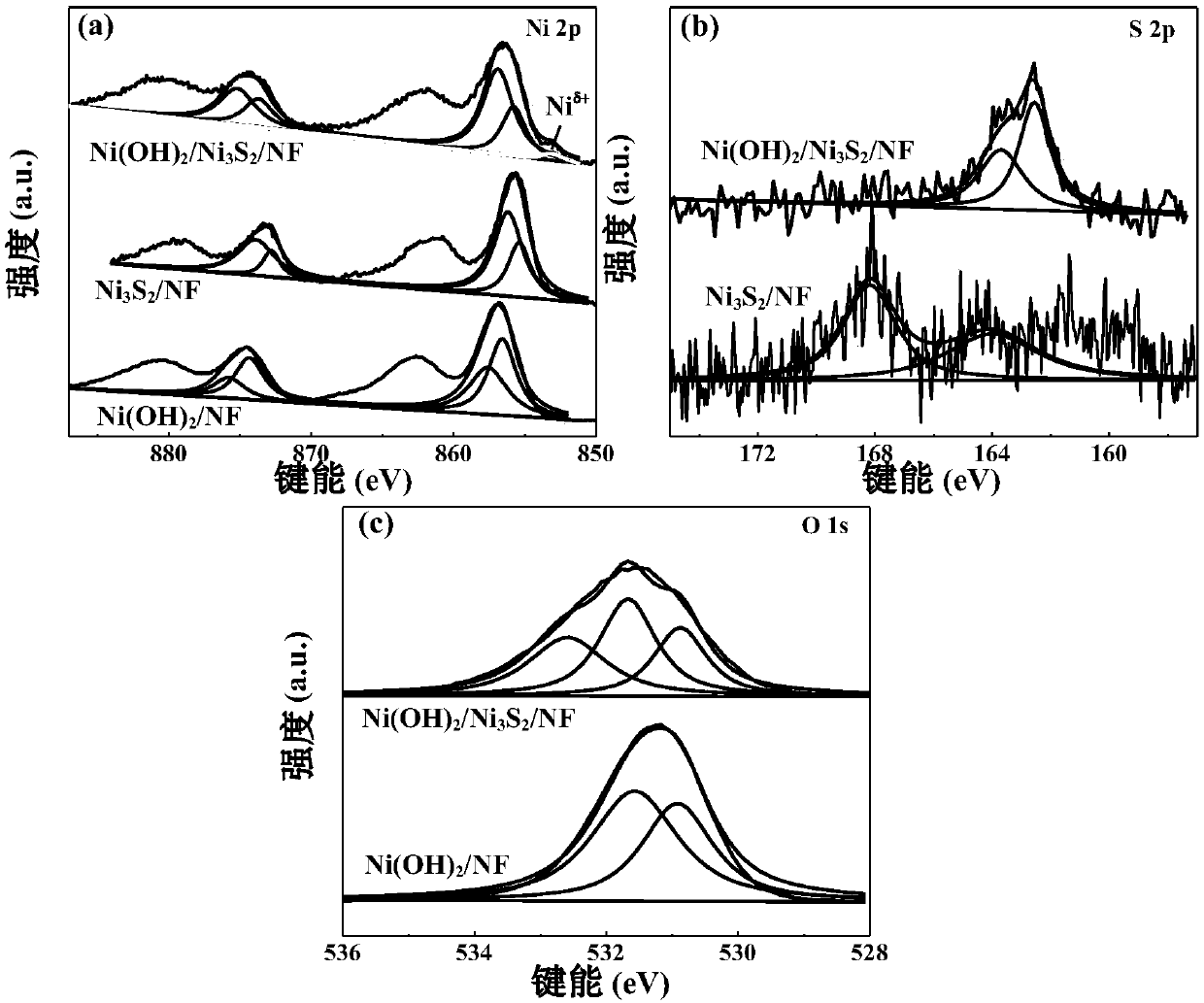
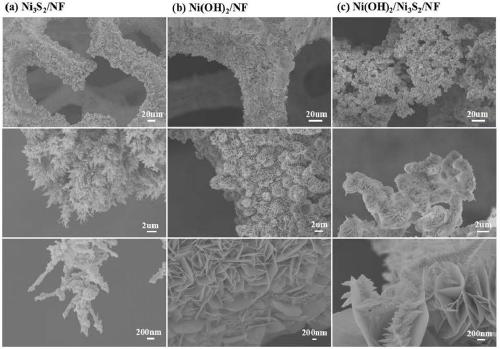
Nickel hydroxide/nickel disulfide/foam nickel composite and preparation method thereof, and application thereof
ActiveCN109659143AAbundant raw materialsLow costMaterial nanotechnologyPhysical/chemical process catalystsElectrolysisSynthesis methods
The invention discloses a nickel hydroxide / nickel disulfide / foam nickel composite and a preparation method thereof, and application thereof. The composite is expressed in the form of Ni(OH)2 / Ni3S2 / NFand can be used as an electrode. In the electrode structure, the nano-stem-like Ni3S2 / NF is a conductive skeleton, and the Ni(OH)2 nanosheet is equivalent to a leaf and grows on the nano-stem-like Ni3S2 / NF skeleton. The heterointerface of Ni(OH)2 and Ni3S2 having a defect structure is the active center. The Ni(OH)2 / Ni3S2 / NF electrode can be used as a bifunctional catalyst, has excellent cathodic catalytic hydrogen evolution and anodic catalytic oxygen evolution reaction performance, and has high current long-term total electrolysis hydrogen production and oxygen production stability. Comparedwith noble metal catalysts such as Pt and RuO2, the catalyst synthesis method is simple, rich in raw materials and low in cost.
Owner:JINAN UNIVERSITY
Supercapacitor electrode material and preparation method thereof
ActiveCN107785181AImprove cycle performanceImprove Capacitive PerformanceHybrid capacitor electrodesHybrid/EDL manufactureNickel oxide hydroxideCapacitance
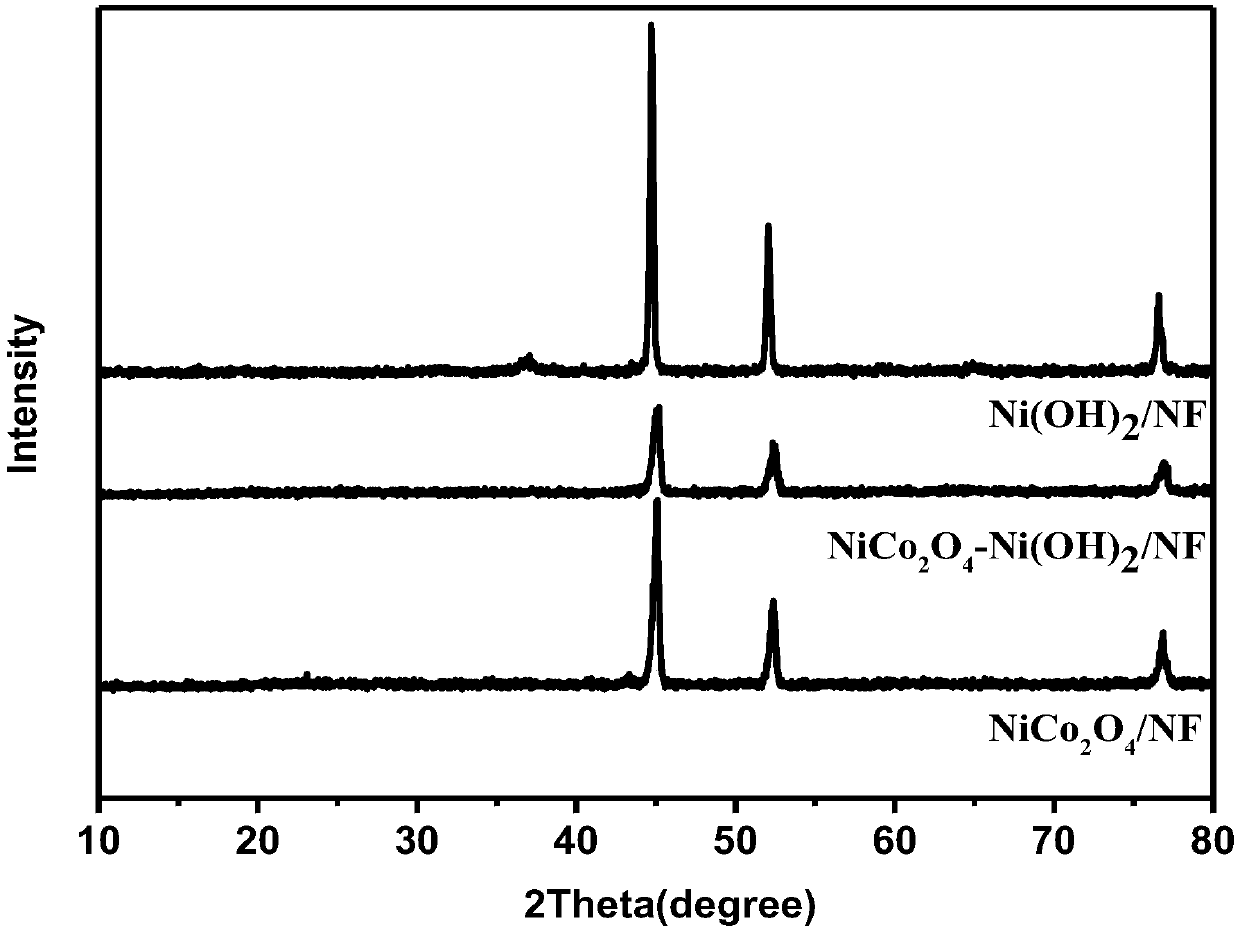
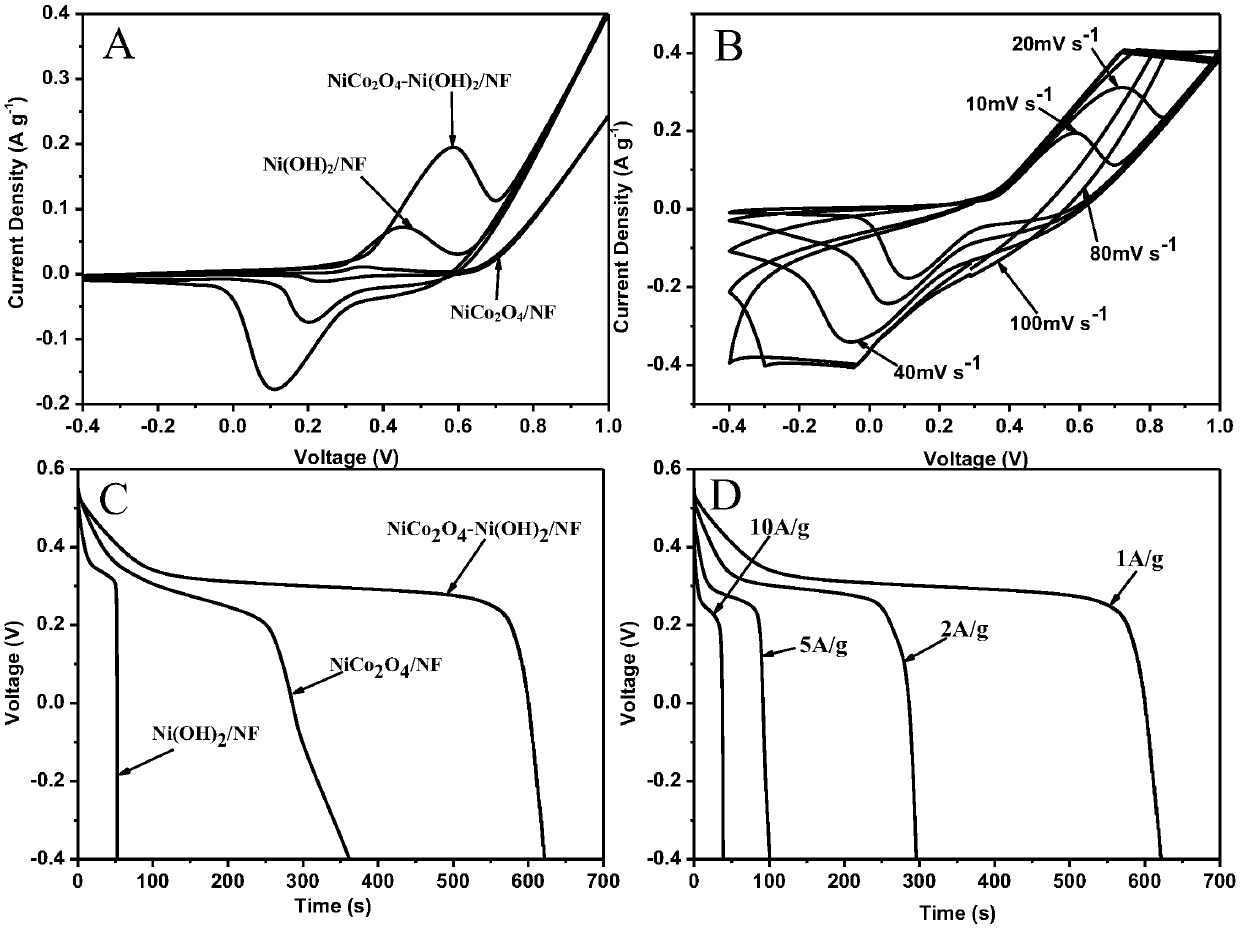
The invention belongs to the technical field of preparation of supercapacitor materials, and relates to a preparation method of a nickel cobaltate / nickel hydroxide / foamed nickel supercapacitor electrode material. According to the method, the nickel cobaltate is enabled to grow on the foamed nickel in situ, and then the nickel hydroxide is electrochemically deposited on a nickel cobaltate / foamed nickel sample. Meanwhile, the high specific surface area of the sample is maintained. During the rapid charging and discharging process, the nickel hydroxide / nickel cobaltate / foamed nickel composite electrode material is maintained to be high in cycling performance and high in capacitance performance. The nickel hydroxide / nickel cobaltate / foamed nickel electrode material can be directly subjected toelectrochemical test, and is different from the traditional sample dropping test.
Owner:合肥九州龙腾科技成果转化有限公司
Multi-level structure alpha type nickel hydroxide prepared by microwave auxiliary and method thereof
InactiveCN101618895AMaterials are cheap and readily availableEasy to operateIndividual molecule manipulationNickel oxides/hydroxidesSolventUrea
The invention relates to multi-level structure alpha type nickel hydroxide prepared by microwave auxiliary and a method thereof. Soluble nickel salt as a raw material, urea as precipitator and mixed solution of ultrapure water and ethanol as solvent are stirred and mixed at room temperature to form uniform solution; and the solution is transferred to a microwave reaction quartz flask, and a microwave device is set to react in a temperature range of between 100 and 180 DEG C. After the reaction is finished, products are separated, washed and dried to obtain alpha type nickel hydroxide nanopowder. The shape of the product can be controlled through changing the microwave reaction temperature, time, and type of the nickel salt raw material, volume ratio of the ultrapure water and the ethanol, and other preparation conditions. The method has the characteristics of low cost of the raw material, simple process, convenient operation, quick and high efficient reaction, controllable shape and the like.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Ferronickel hydroxide/reduction-oxidation graphene electrochemical oxygen evolution catalyst with nickel foam as carrier and preparation method of ferronickel hydroxide/reduction-oxidation graphene electrochemical oxygen evolution catalyst
InactiveCN108707923AHigh activityImprove stabilityLiquid/solution decomposition chemical coatingElectrode shape/formsIron saltsNickel salt
The invention relates to a ferronickel hydroxide / reduction-oxidation graphene electrochemical oxygen evolution catalyst with nickel foam as a carrier and a preparation method of the ferronickel hydroxide / reduction-oxidation graphene electrochemical oxygen evolution catalyst. The nickel foam is used as the carrier of the catalyst, ferric hydroxide, nickel hydroxide or a compound of the ferric hydroxide and the nickel hydroxide is used as the active component of the catalyst, and reduction-oxidation graphene is used as the conductive material of the catalyst. The catalyst is prepared through a water heating and impregnation method. Firstly, the nickel foam is subjected to ultrasonic cleaning and drying; then, a certain amount of nickel salt and a certain amount of urea are weighed, dissolvedin deionized water and stirred to be evenly mixed at the room temperature; then, oxidized graphene is added, ultrasonic treatment is conducted, and evenly-dispersed mixed liquor is obtained; the nickel foam and the mixed liquor are transferred into a water heating kettle and react for 12 h to 24 h at the temperature being 120-200 DEG C; the nickel foam subjected to reaction is cleaned and dried;and finally, after the nickel foam is soaked in an iron salt solution with the concentration ranging from 5 mmol / L to 30 mmol / L for 0 h to 48 h, cleaning and drying are conducted, and the ferronickelhydroxide / reduction-oxidation graphene electrochemical oxygen evolution catalyst with the nickel foam as the carrier is obtained. The catalyst is good in catalysis activity and high in stability; andthe preparation method is simple and controllable, and industrial popularization is facilitated.
Owner:EAST CHINA UNIV OF SCI & TECH
Composite material of nickel hydroxide/graphene or graphite and preparation method for composite material
InactiveCN104616908AIncrease volumetric energy densityLow costMaterial nanotechnologyHybrid capacitor electrodesGraphiteGraphene
The invention discloses a composite material of nickel hydroxide / graphene or graphite which is structurally Ni(OH)2 / graphite or layered Ni(OH)2 / graphene composite material alternated with Ni(OH)2 and graphene, wherein the weight content of the nickel hydroxide is 10-90%. The invention further discloses a method for preparing the composite material. The composite material is simple in process, low in cost, environment-friendly and easy for batch production.
Owner:SOUTHWEST PETROLEUM UNIV
Foamed nickel self-supported flake-shaped Ni3P/C composite material for sodium ion battery negative electrode and preparation method for composite material
ActiveCN105720236AImprove performanceExcellent rate performanceNegative electrodesCarbon coatingThermal insulation
The invention discloses a foamed nickel self-supported flake-shaped Ni3P / C composite material for a sodium ion battery negative electrode and a preparation method for the composite material. According to the composite material, the flake-shaped Ni3P is uniformly growing on the foamed nickel; and the Ni3P is uniformly coated with a C film. The preparation method for the composite material comprises the steps of taking a nickel compound as the raw material, and uniformly growing a flake-shaped nickel hydroxide layer on the surface of the nickel compound through a hydrothermal method; then taking sodium hypophosphite as a phosphorus source, and performing thermal insulation at a temperature of 300 DEG C for 2h to prepare the foamed nickel self-supported flake-shaped Ni3P material; and finally, performing carbon coating on the foamed nickel self-supported flake-shaped Ni3P material to obtain the foamed nickel self-supported flake-shaped Ni3P / C composite material. The sodium ion battery prepared from the Ni3P / C composite material prepared by the invention has excellent specific capacity, rate capability and stable cycling performance; and in addition, the preparation method is simple and feasible, wide in raw material resources and suitable for industrial production.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing nanometer nickel oxide and application of method
InactiveCN102603016ASimple processEasy to operateNickel oxides/hydroxidesMaterial electrochemical variablesElectrolytic agentElectrochemistry
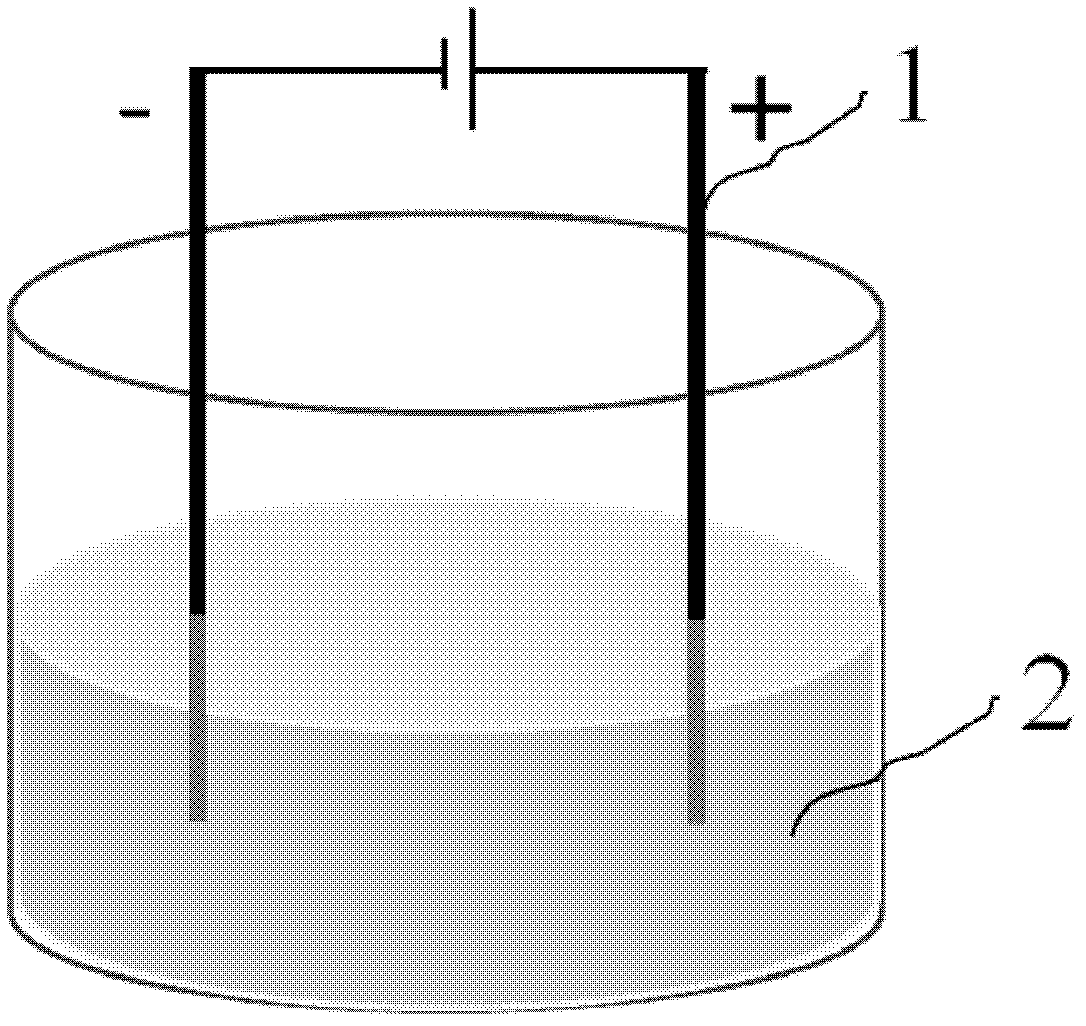
The invention discloses a method for preparing nanometer nickel oxide and application of the method. The method includes adding a certain quantity of inorganic salt and organic liquor into aqueous liquor to be used as electrolyte; using metal nickel as an electrode; applying voltage to two ends of the electrode; forming nickel hydroxide nano-particles by means of regulating the concentration of the electrolyte, the amplitude of the voltage, the reaction time and the stirring speed; then annealing the nickel hydroxide nano-particles at the temperature of 300 DEG C; and obtaining the nickel hydroxide nano-particles. A formaldehyde sensor built on the basis of the nanometer nickel oxide can be used for detecting formaldehyde gases with different concentrations. The method has the advantages that the nanometer nickel oxide is prepared in the electro-chemical method the nanometer nickel oxide is used for building the formaldehyde sensor, a process is simple, operation is convenient, environmental pollution is prevented, the cost of a product is low, repeatability is good, and the method is applicable to large-scale industrial production.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
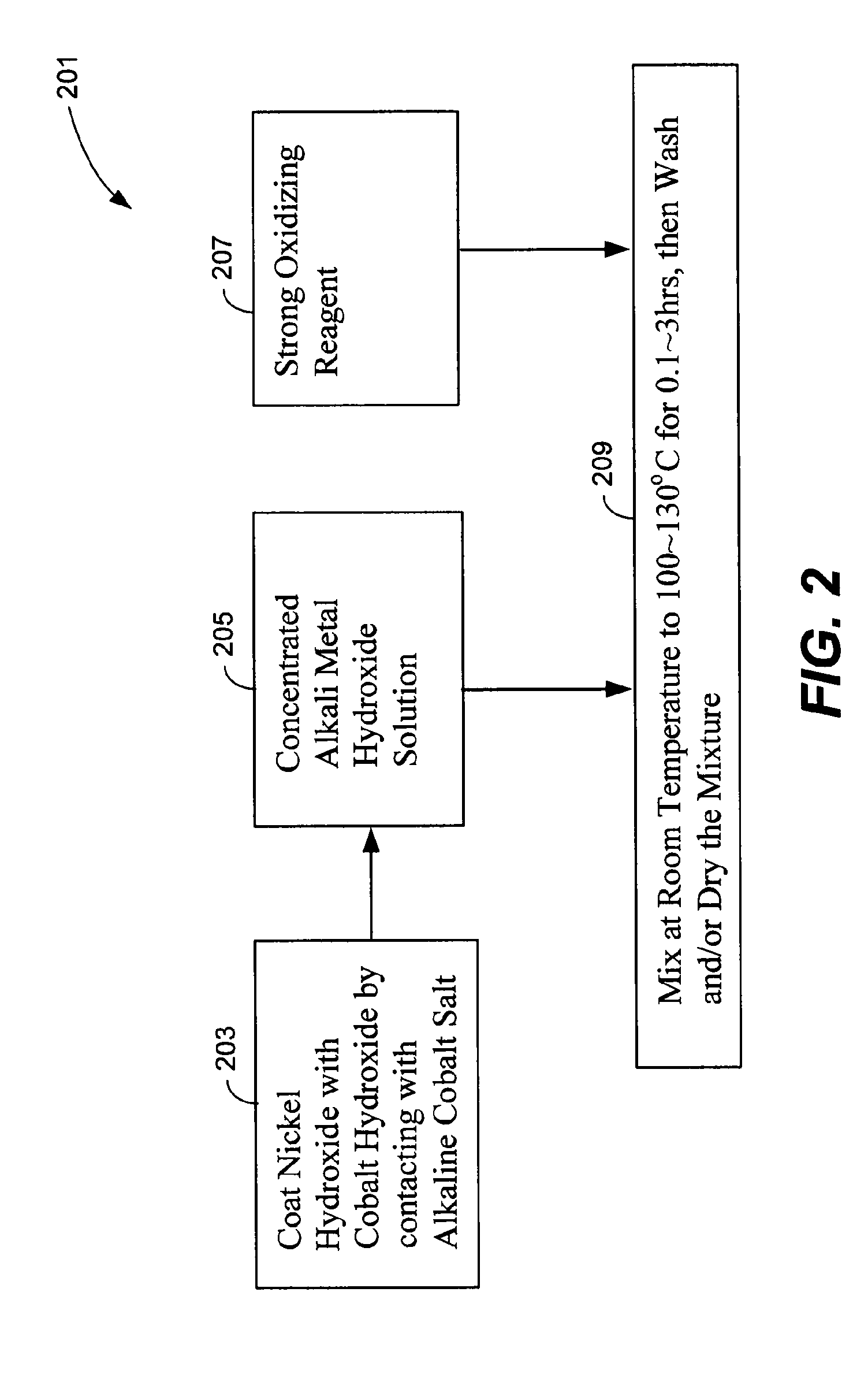
Preparation method of spherical nickel hydroxide anode material coated with gamma-hydroxy cobalt oxide
InactiveCN102142547AImprove liquidityFully dispersedAlkaline accumulator electrodesLithium hydroxidePhysical chemistry
The invention relates to a preparation method of a spherical nickel hydroxide anode material coated with gamma-hydroxy cobalt oxide, comprising the following steps: heating cobalt hydroxide coated spherical nickel hydroxide which is utilized as a precursor, and spraying an alkali metal hydroxide aqueous solution; evenly mixing the precursor with the alkali metal hydroxide aqueous solution; introducing oxygen to oxidize the cobalt hydroxide to convert the cobalt hydroxide into high-valency gamma-hydroxy cobalt oxide; and during oxidation, combining mechanical agitation and ultrasonic agitation to prevent the spherical nickel hydroxide anode material coated with gamma-hydroxy cobalt oxide from gathering and cause alkali metal ions to be embedded into a crystal lattice with the laminated structure of the gamma-hydroxy cobalt oxide. After oxidation reaction is over, a lithium hydroxide alkaline solution at certain temperature is added into an oxidation reaction pot to carry out heat preservation on the spherical nickel hydroxide anode material coated with gamma-hydroxy cobalt oxide; when heat preservation is carried out, mechanical agitation and ultrasonic agitation are adopted to cause the lithium ions to be embedded into the laminated structure of the gamma-hydroxy cobalt oxide; and the obtained spherical nickel hydroxide anode material coated with gamma-hydroxy cobalt oxide is screened by an ultrasonic sieve shaker after being washed and dried.
Owner:JIANGMEN CHANCSUN UMICORE IND
Purification method of cobalt nickel hydroxide hydrochloric acid leaching solution
ActiveCN104073633AImprove qualityReduce usageProcess efficiency improvementPregnant leach solutionSulfate radicals
The invention discloses a purification method of a cobalt nickel hydroxide hydrochloric acid leaching solution. The purification method mainly comprises the following processing steps: I. dissolving and leaching a cobalt nickel hydroxide raw material produced by laterite-nickel ore transformation through industrial concentrated hydrochloric acid; II. adding barium chloride to a leaching solution and implementing sulfate radical removal operation; filter-pressing the solution and reserving the solution as an extracting pre-solution for later use; III. preparing an organic phase at ratio of 25% to 75% of P5O7 to sulfonated kerosene; saponifying the prepared organic phase in a soda soap kettle; reacting the saponified organic phase with a pure nickel chloride solution to obtain nickel soap; and IV. extracting the nickel chloride extracting pre-solution obtained from the step II through the P507 nickel soap, wherein the extracting solution obtained is a finished pure nickel chloride solution. The production process disclosed by the invention is safe and the extracting solution produced is better in component; and moreover, the purification method is short in technological process and more effective in process control.
Owner:JINCHUAN GROUP LIMITED
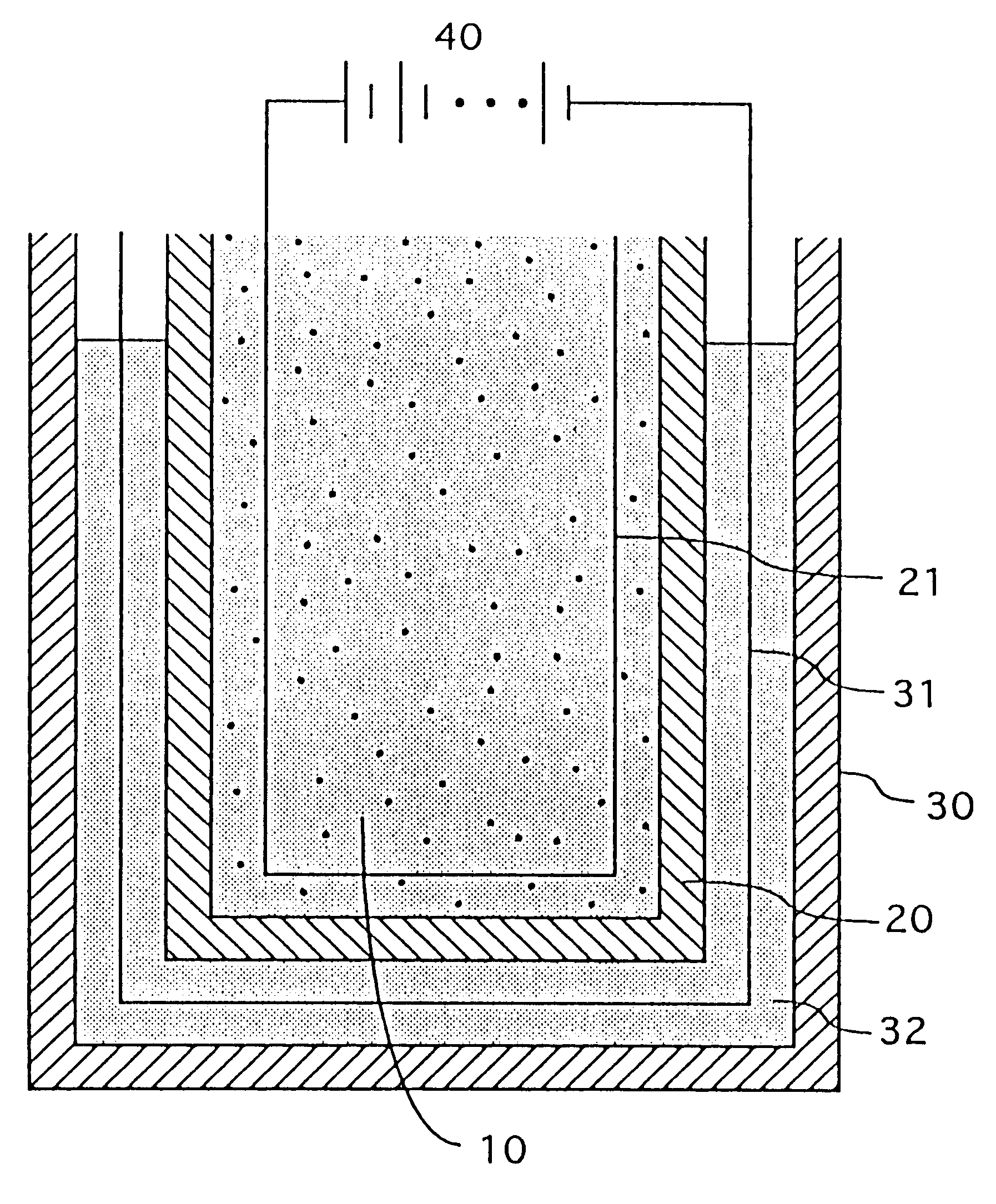
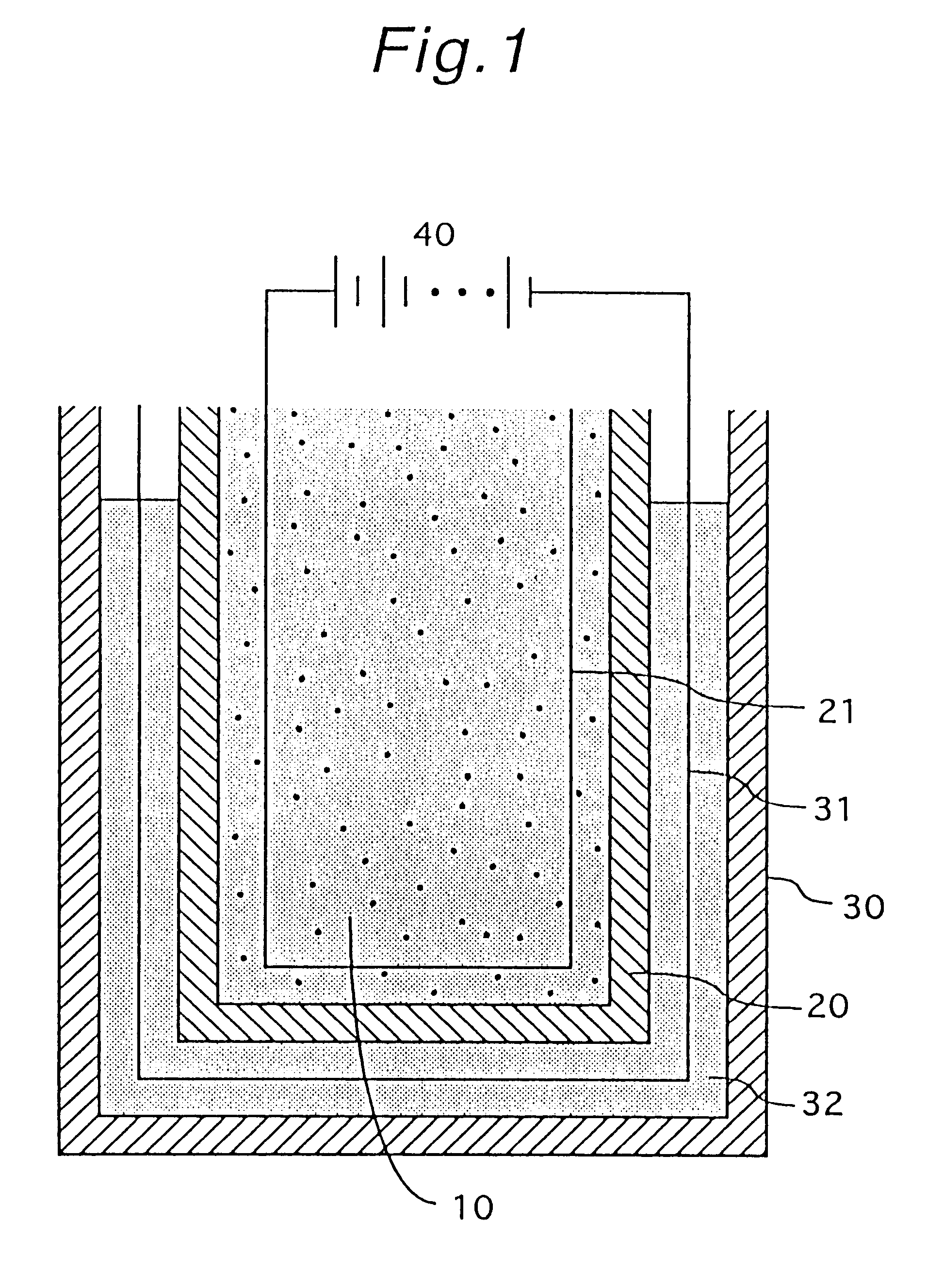
Nickel electrode and alkali storage battery using the same
InactiveUS20060029864A1Improve performanceHigh tap densityAlkaline accumulatorsElectrode carriers/collectorsPhysical chemistryNickel electrode
An alkali storage battery using powder generation elements composed of a positive electrode comprising nickel (Ni) oxide as main materials, a negative electrode, a separator and an alkali aqueous solution, wherein materials of said positive electrodes are spherical or elliptic powders whose tapping density is not less than 2.2 g / cc mainly composed of nickel hydroxide (Ni(OH)2), powders comprise core powders with innumerable microscopic concaves and convexes on surfaces mainly composed of spherical or elliptic β-type Ni(OH)2, and fine powders composed of metal cobalt (Co) and / or Co oxide, and fine powders are crushed and pressed in substantially all concave portions of microscopic concaves and convexes of said core powders, thereby integrated with core powders, and surface layers of powders are coated with fine powders and are flattened, and core powders and / or fine powders have innumerable micro pores which penetrate from surfaces to inner portions.
Owner:M&G ECO BATTERY INST
Paste type positive electrode for alkaline storage battery, and nickel-metal hydride storage battery
InactiveUS6858347B2Low cycle lifeReduce capacityFinal product manufactureAlkaline accumulator electrodesParticulatesNickel oxide hydroxide
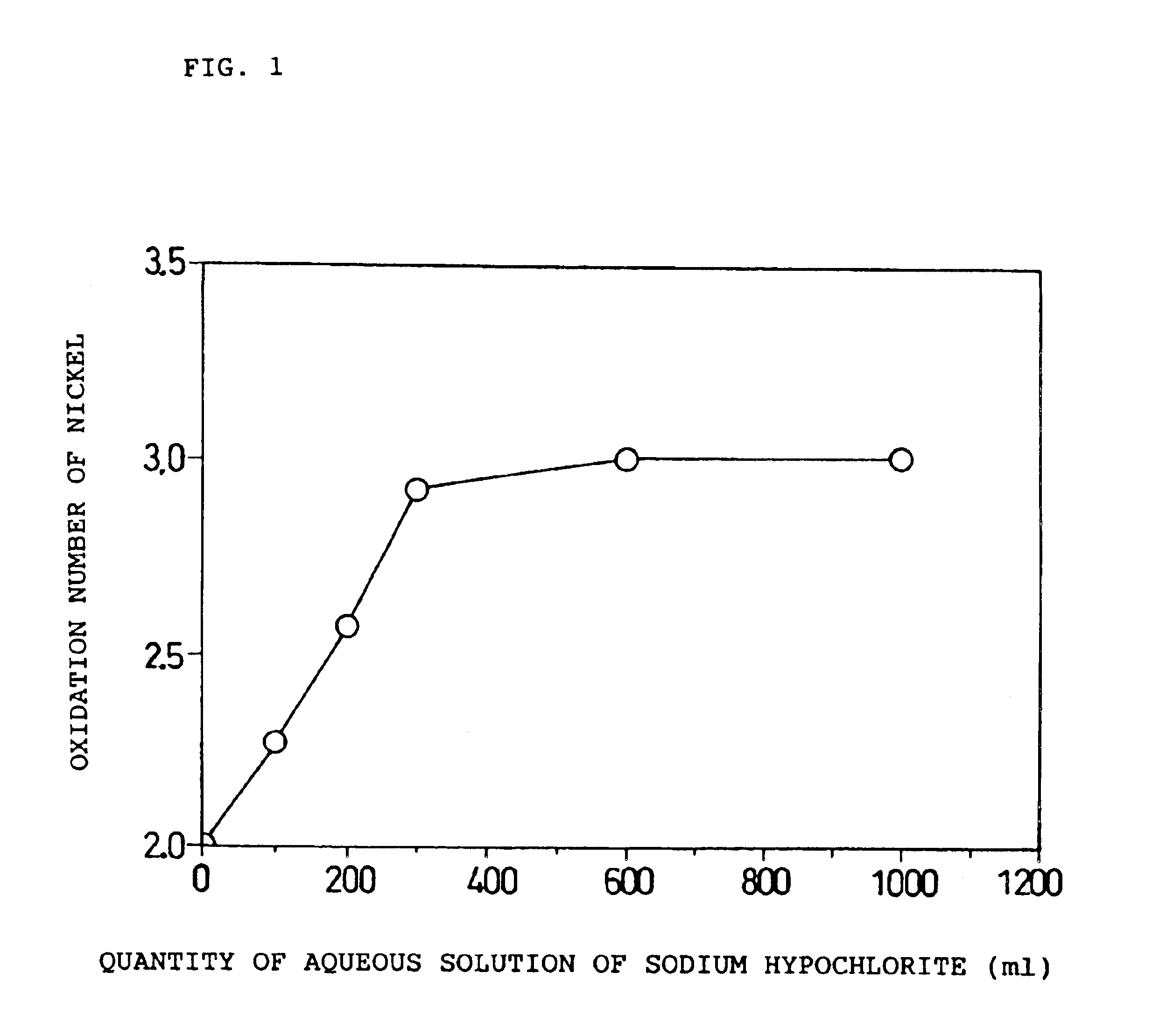
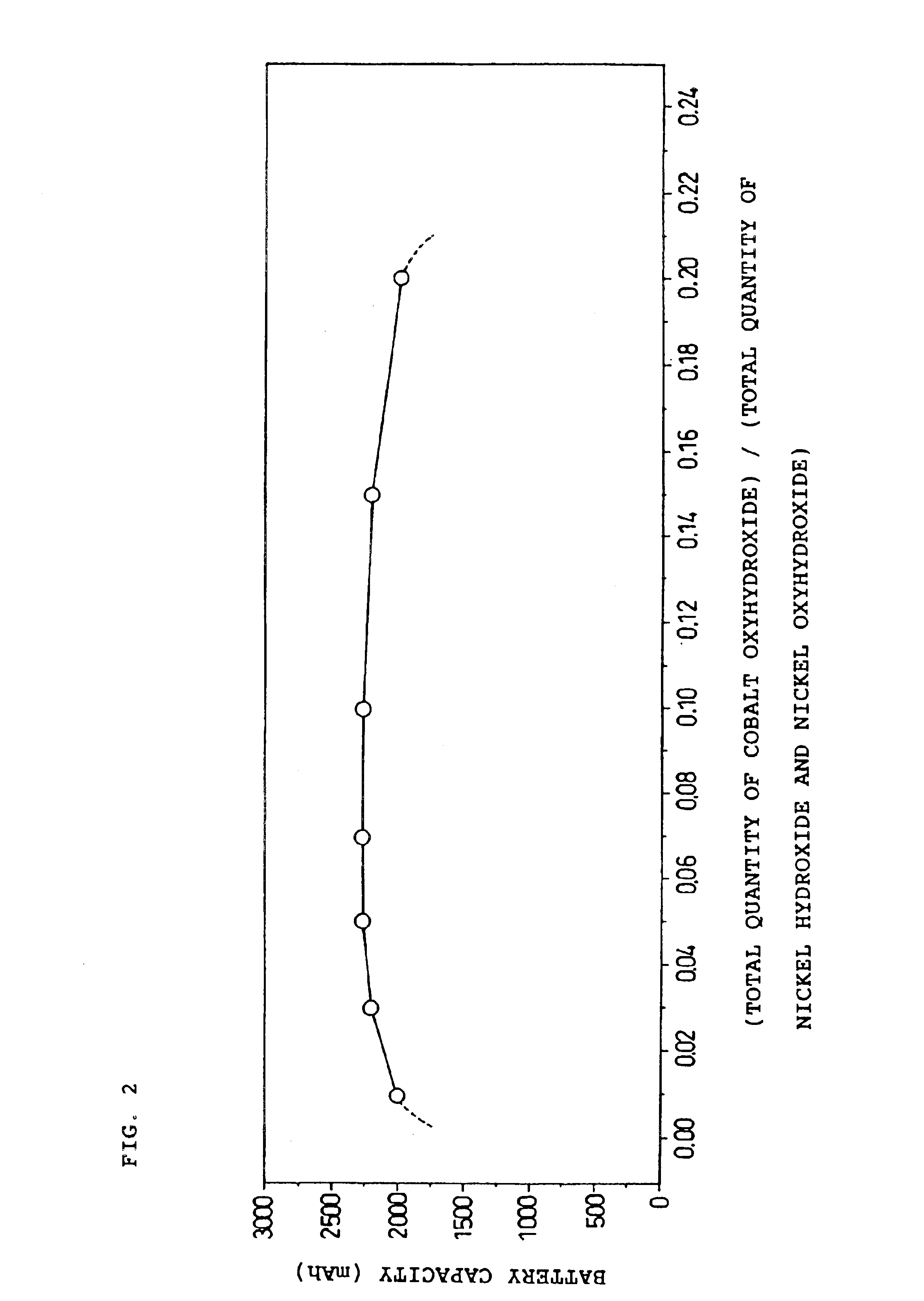
The paste type positive electrode of the present invention contains a first active material and a second active material. The first active material comprises X parts by weight of particulate nickel hydroxide with aX / 100 parts by weight of cobalt oxyhydroxide carried thereon. The second active material comprises Y parts by weight of particulate nickel oxyhydroxide, of which an oxidation number of nickel is α, with bY / 100 parts by weight of cobalt oxyhydroxide carried thereon. Here, all the following relations are satisfied: (1) 2.5≦α<3.0, (2) 0.01≦(aX / 100+bY / 100) / (X+Y)≦0.20, (3) 0<b≦a≦10 or 0=b<a≦10, and (4) 2.1≦(2X+αY) / (X+Y)<2.2.
Owner:PANASONIC CORP
Method for preparing nano nickel bicarbonate
The invention relates to a method for preparing nano nickel bicarbonate, which comprises the following steps of: adding water into urea or urotropine for dissolving, adding nickel salt or nickel hydroxide and stirring, wherein the mole ratio of the urea or the urotropine to nickel is 1:1-16:1, and the concentration of the nickel salt in solution is 0.1-1.0mol / L; shifting the obtained solution into a hydrothermal reactor; after reacting at 90-240 DEG C for 1-96 hours, cooling; and filtering obtained reaction mixed liquid, washing with distilled water and anhydrous ethanol and placing in a vacuum drying box at 60 DEG C for drying to obtain a cubic phase nano nickel bicarbonate square block with the size of 100nm or so. The invention uses the urotropine or the urea as a precipitator, NH3 andCO2 generated by the precipitator dissolve nickel hydroxide, and the pure cubic phase Ni(HCO3)2 nano crystal is prepared finally.
Owner:NANJING UNIV OF TECH
Method for producing nickel carbonyl powder from nickel hydroxide
ActiveCN103130284AMild reaction conditionsSimple processNickel carbonylsDecomposerNickel oxide hydroxide
The invention relates to a method for producing nickel carbonyl powder from nickel hydroxide, belonging to the technical field of powder metallurgy. The method comprises the following steps: adding nickel hydroxide into a rotary roasting furnace, removing water at high temperature, and roasting to obtain nickel oxide; adding the oxide into a hydrogen reducing furnace to perform reduction, thereby generating simple substance active nickel; adding the simple substance active nickel into a nickel carbonyl synthesis reactor to make counterflow contact with CO, and reacting to generate nickel carbonyl gas; delivering the nickel carbonyl gas to a nickel carbonyl decomposer by a blower, and delivering generated nickel carbonyl residues into a nickel carbonyl treater to perform passivating treatment; and decomposing the nickel carbonyl gas in the nickel carbonyl decomposer to produce the nickel carbonyl powder. The invention does not need to perform extra treatment on the raw material, and has the advantages of mild reaction conditions, simple technique, high production safety, low cost, high synthesis efficiency and the like.
Owner:吉林卓创新材料有限公司
Self-collecting supercapacitor electrode material and preparing method thereof
ActiveCN103762090AReduce productionIncrease specific volumeHybrid capacitor electrodesNanotechnologyMaterials scienceHydrogen peroxide
The invention provides a self-collecting supercapacitor electrode material and a preparing method thereof. The electrode material is composed of a foamed nickel current collector and a b-nickel hydroxide hexagonal nanosheet grown on the surface of the foamed nickel current collector in an in-situ mode. According to the preparing method of the electrode material, the conductive current collector foamed nickel is immersed into a hydrogen peroxide solution for low-temperature hydrothermal oxidation, and then the b-nickel hydroxide hexagonal nanosheet is directly grown on the conductive current collector in the in-situ mode. The electrode material has the advantages of being large in specific volume and good in circulating stability. Due to the fact that the active material nickel hydroxide is directly grown on the foamed nickel current collector, the electrode material can be used directly without extra current collectors, or conductive additives or binding agents, complicated electrode preparing processes are omitted, and self-collecting is achieved. The electrode material preparing method only relates to the cheap hydrogen peroxide solution, other chemical reagents are not needed, and then zero pollution and low cost are guaranteed. The preparing processes only relate to hydrothermal oxidation and vacuum drying, operation is easy and convenient, reproducibility is good, and bulk preparation and industrialized production are facilitated.
Owner:CHONGQING UNIV
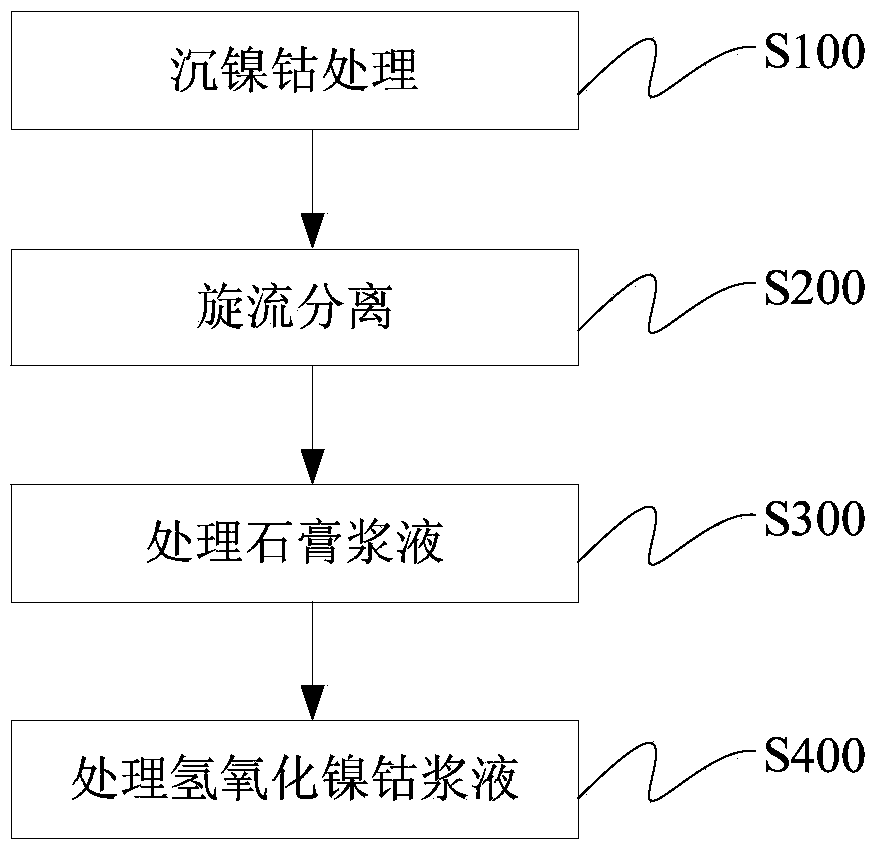
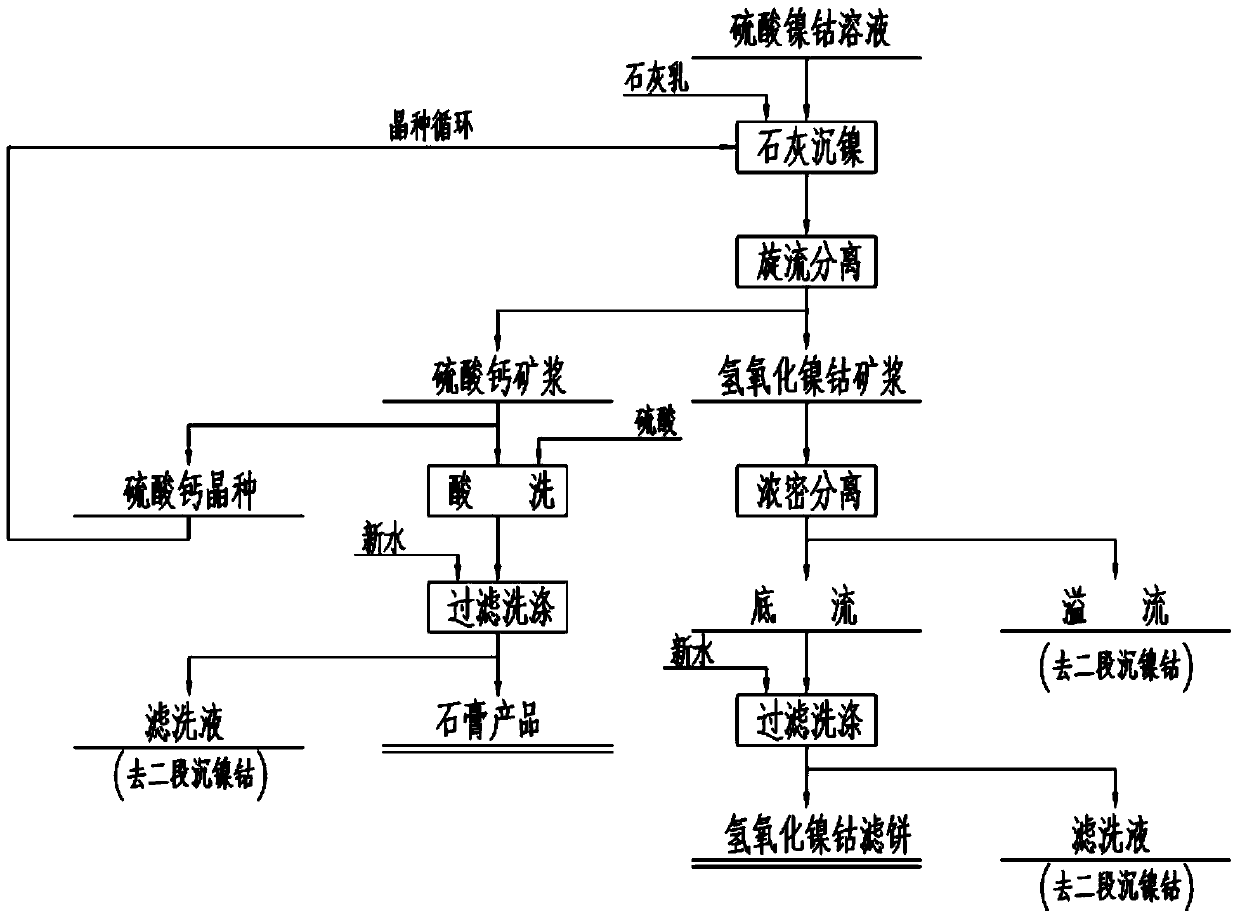
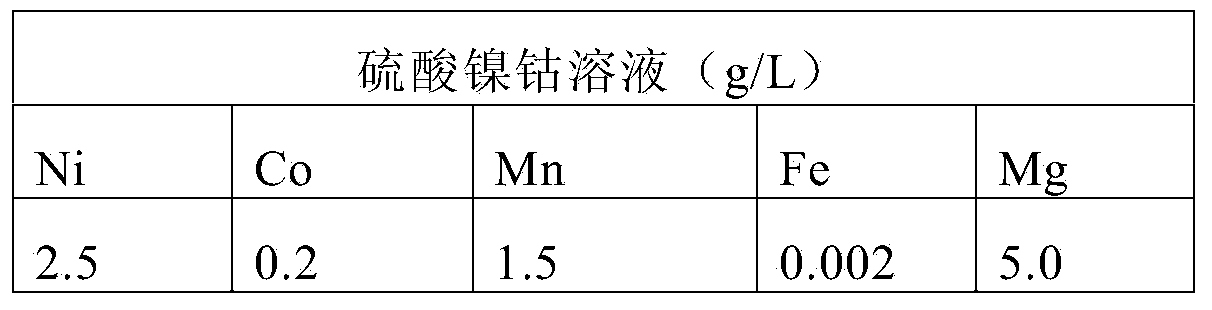
Method for treating cobalt nickel sulfate solution
The invention discloses a method for treating a cobalt nickel sulfate solution. The method comprises the steps of (1) adding lime milk into the cobalt nickel sulfate solution to carry out nickel and cobalt sedimentation treatment to obtain cobalt nickel hydroxide sediment and gypsum contained mixed slurry; (2) carrying out hydrocyclone separation on the mixed slurry to obtain cobalt nickel hydroxide slurry and gypsum slurry; (3) pickling and filtering the gypsum slurry to obtain gypsum and first filtrate; (4) thickening and separating the cobalt nickel hydroxide slurry to obtain underflow slurry and overflow slurry; (5) cleaning and filtering the underflow slurry to obtain a cobalt nickel hydroxide filter cake and second filtrate. By using the method, the treatment cost can be effectively reduced, and other impurities can be prevented form being introduced.
Owner:CHINA ENFI ENGINEERING CORPORATION
Preparation method of flower-like alpha-nickel hydroxide
The invention discloses a preparation method of flower-like alpha-Ni(OH)2. The preparation method comprises the following steps of: adding a nickel salt and urotropine into de-ionized water, stirring and dissolving, wherein the amount ratio of the nickel to the urotropine is 1:1-1:2, and the concentration of the nickel salt in the solution is 0.1-1 mol / L; taking 5 mL of mixed solution; adding 10-35 mL of lower alcohol into the mixed solution; adding the obtained solution into de-ionized water until the volume is 40 mL; transferring to 50 mL polytetrafluoroethylene-lined high pressure reaction kettle; reacting at 160 DEG C for 2 hours; cooling; filtering; washing with the de-ionized water and absolute ethyl alcohol for many times to filter a product respectively; an holding in a vacuum drying box of 60 DEG C for drying to prepare the flower-like alpha-Ni(OH)2. According to the preparation method, the urotropine is used as a precipitator, the ethanol is used as a solvent, and the finally purer flower-like alpha-Ni(OH)2 is prepared.
Owner:NANJING UNIV OF TECH
Bismuth ferrite/nickel hydroxide secondary alkali battery and preparation method therefor
InactiveCN106654401AHigh specific capacityImprove cycle stabilityCell electrodesFinal product manufactureElectrical batteryMaterials science
The invention discloses a bismuth ferrite / nickel hydroxide secondary alkali battery and a preparation method therefor. Bismuth ferrite (BiFeO<3>) is used as a negative electrode material of the battery; nickel hydroxide is used as a positive electrode material of the battery; an alkali solution is used as an electrolyte solution; and the battery is 0.2-1.5V in voltage window. The preparation method for the bismuth ferrite comprises the following steps of enabling a mixed solution of bismuth and iron ions and a precipitator solution to be reacted based on certain stoichiometric ratio to obtain a precursor; and putting the precursor into a high-temperature furnace under certain atmosphere environment, performing heat treatment to obtain a product, and then performing washing, solid / liquid separation, drying and grinding on the product to prepare the bismuth ferrite material. The specific capacity of the configured battery is 243mAh / g at current density of 1A / g; and the capacity retention rate is 78.6% after 500 circles. The battery is high in electrochemical performance and environment-friendly, and is a novel reversible secondary chemical power supply with wide application prospect.
Owner:XIANGTAN UNIV
Alkaline storage battery
InactiveUS20040146782A1Improve battery lifePrevent oxidationAlkaline accumulator electrodesNon-aqueous electrolyte accumulator electrodesRare-earth elementElectrolyte
An alkaline storage battery having a negative electrode made from a hydrogen absorbing alloy represented by the formula Ln1-xMgxNiy-aMa (where Ln is at least one element selected from rare earth elements, M is at least one element selected from the group consisting of Al, V, Nb, Ta, Cr, Mo, Mn, Fe, Co, Ga, Zn, Sn, In, Cu, Si and P, 0.05<=x<0.20, 2.8<=y<=3.9 and 0.10<=a<=0.50) and carbon as a conductive agent, a positive electrode of nickel hydroxide as an active material, and an alkaline electrolyte, and the alkaline storage battery contains not greater than 0.01 weight % of hydrogen or not greater than 0.13 weight % of water in the hydrogen absorbing alloy when the battery is activated and is discharged to 1.0 V at one hour rate (It).
Owner:SANYO ELECTRIC CO LTD
Nickel series rechargeable battery and its producing method
InactiveCN1339844AIncrease profitIncrease energy densityMaterial nanotechnologyFinal product manufactureParticulatesCrystallography
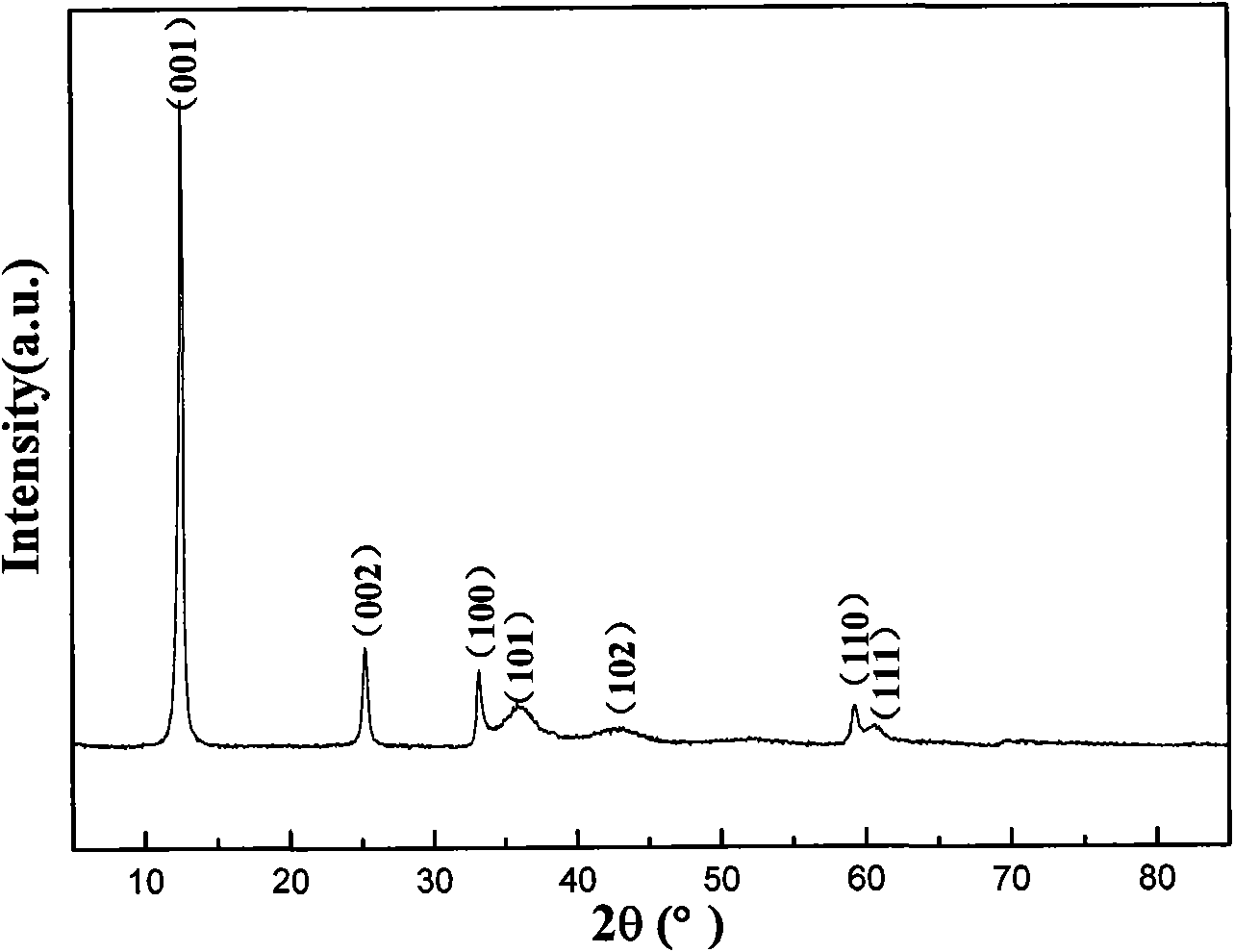
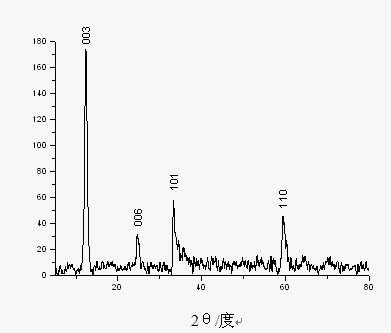
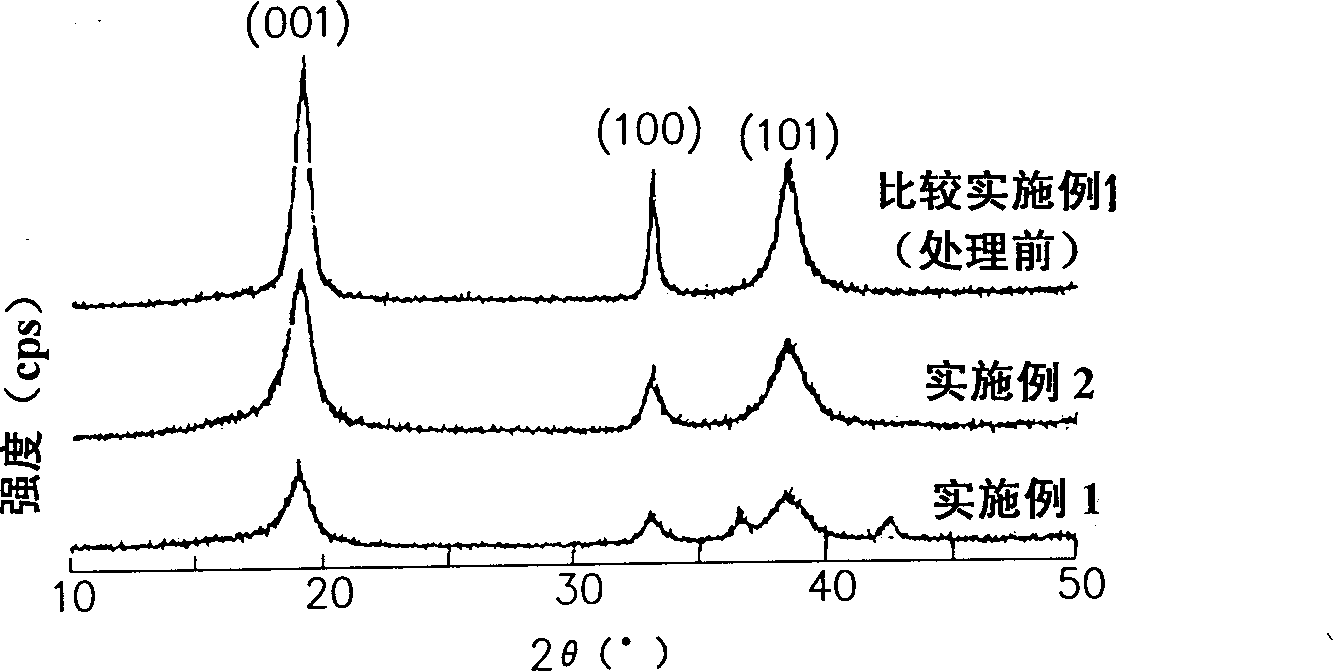
A nickel-series rechargeable battery whose cathode active material comprising a material containing an amorphous phase-bearing nickel hydroxide particulate which in X-ray diffraction using K alpha -rays of Cu as a radiation source, has a diffraction peak of a (001) face appeared near a diffraction angle 2 theta = 19 DEG having a half-value width of more than 1.2 DEG and has a diffraction peak of a (101) face appeared near a diffraction angle 2 theta = 38 DEG having a half-value width of more than 1.5 DEG . A process for the production of said rechargeable battery is also provided.
Owner:CANON KK
Bismuth/nickel hydroxide secondary alkaline battery and preparation method thereof
ActiveCN106450249ALarge capacityImprove cycle lifeCell electrodesFinal product manufacturePower flowElectric capacity
The invention discloses a bismuth / nickel hydroxide secondary alkaline battery and a preparation method thereof. The battery provided by the invention takes metal bismuth as a negative electrode active material of the battery, nickel hydroxide as a positive electrode active material of the battery and an alkaline solution as an electrolyte solution, and a voltage window of the battery is 0.2V to 1.4V. Preparation of a bismuth material comprises: dropwise adding a reducing agent solution into a bismuth ion solution containing an auxiliary agent according to a certain mol ratio of a reducing agent to bismuth ions, and reacting for a certain time; after the reaction is finished, washing a product and carrying out solid-liquid separation; drying solids in vacuum to prepare the bismuth material. The bismuth material is metal powder with the grain diameter of 0.005mu m to 5mu m, and the specific surface area of the bismuth material is 5m<2> / g to 500m<2> / g. The specific capacity of the battery under the current density of 5A / g is 254mAh / g. The battery provided by the invention has large electric capacity and good cycling stability, is environment-friendly and is a novel chemical power supply with a wide application prospect.
Owner:XIANGTAN UNIV
Nickel oxide powder and preparation method thereof
The invention relates to solid oxide powder and a preparation method thereof, in particular to nickel oxide powder and a preparation method thereof. The preparation method of the nickel oxide powder disclosed by the invention comprises the following steps of (1) mixing a nickel salt solution with a complexing agent, and performing stirring so as to obtain a complex compound solution, wherein the mole ratio of nickel ions contained in the nickel salt solution to donor atoms in the complexing agent is (1 to 5) to (1 to 6); (2) adding a strongly alkaline solution to the complex compound solution obtained in the step (1), performing stirring to obtain nickel hydroxide precursor precipitation, and washing the nickel hydroxide precursor precipitation with water so as to obtain paste; (3) drying the paste obtained in the step (2) so as to obtain a product precursor; and (4) roasting the product precursor obtained in the step (3) so as to obtain the nickel oxide powder. The nickel oxide powder prepared by the preparation method disclosed by the invention is free from clumping, tiny in particle size, low in impurity taste, controllable in sulfur taste, and particularly low in sodium taste and chlorine taste, and can be used as a nickel oxide powder material used for electronic components, and electrodes of solid oxide fuel cells.
Owner:CHAOZHOU THREE CIRCLE GRP
Nickel hydroxide active material for use in alkaline storage cell and manufacturing method of the same
InactiveUS6203945B1Alkaline accumulator electrodesNon-aqueous electrolyte accumulator electrodesNickel oxide hydroxideCrystal structure
A nickel hydroxide active material for use in an alkaline storage cell, the active material being essentially composed of nickel hydroxide particles covered with a cobalt compound adhered to their surfaces, wherein the cobalt compound is disordered in a crystal structure and formed in a higher order state containing alkali cation, and wherein the nickel hydroxide is in the form of a higher order nickel compound having an average oxidation value of 2.15 to 2.40.
Owner:FDK CORP
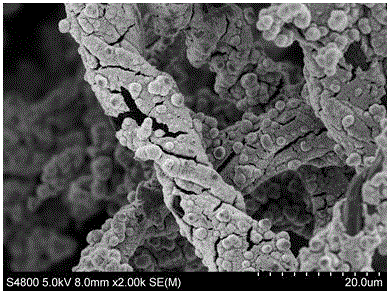
Preparation method and application of carbon fiber based nickel hydroxide composite material
InactiveCN106356201AImprove conductivityReduce clumpingHybrid capacitor electrodesHybrid/EDL manufacturePotassium persulfateFiber
The invention discloses preparation method and application of a carbon fiber based nickel hydroxide composite material. The preparation method includes: subjecting degreasing cotton to high-heat treatment under nitrogen atmosphere to obtain a carbon fiber block; adding nickel sulfate and potassium peroxodisulfate into distilled water for stirring to dissolve, adding the carbon fiber block wetted through methyl alcohol after complete dissolution, under magnetic stirring, dropwise adding 3mL of ammonium hydroxide 28% in mass fraction, and after dropwise adding is completed, stopping stirring for standing for 1 hour; taking a carbon fiber based sample which is washed through the distilled water and then placed in an oven for drying for 12 hours under the temperature of 80DEG C to obtain the carbon fiber based nickel hydroxide composite material which can be used as an electrode material for a supercapacitor. The preparation method and application is simple in operation and easy to implement, and the obtained carbon fiber based nickel hydroxide composite material is good in performance when serving as the electrode material of the supercapacitor.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
In-situ preparation method of multi-grade carbon based iron nickel hydroxide and product thereof and application
ActiveCN108295855AShape is easy to controlSimple equipmentCell electrodesCatalyst activation/preparationNanowirePolypyrrole
The invention relates to an in-situ preparation method of multi-grade carbon based iron nickel hydroxide and a product thereof and application. The method is characteirzed in that a polypyrrole nanowire / needle array grown in-situ on a carbon fabric by an electrochemical method and then is thermally processed to obtain the multi-grade carbon based needle shaped nanowire / needle array utilizing the carbon fabric as a substrate; and then the iron nickel hydroxide is loaded in situ. The method is simple, and low in cost; the prepared multi-grade carbon based nano needle / wire array is high in specific area controllable in appearance, high in oxygen evolution catalyzing performance, and widely applicable to the fields of energy storage and conversion.
Owner:CHONGQING UNIV
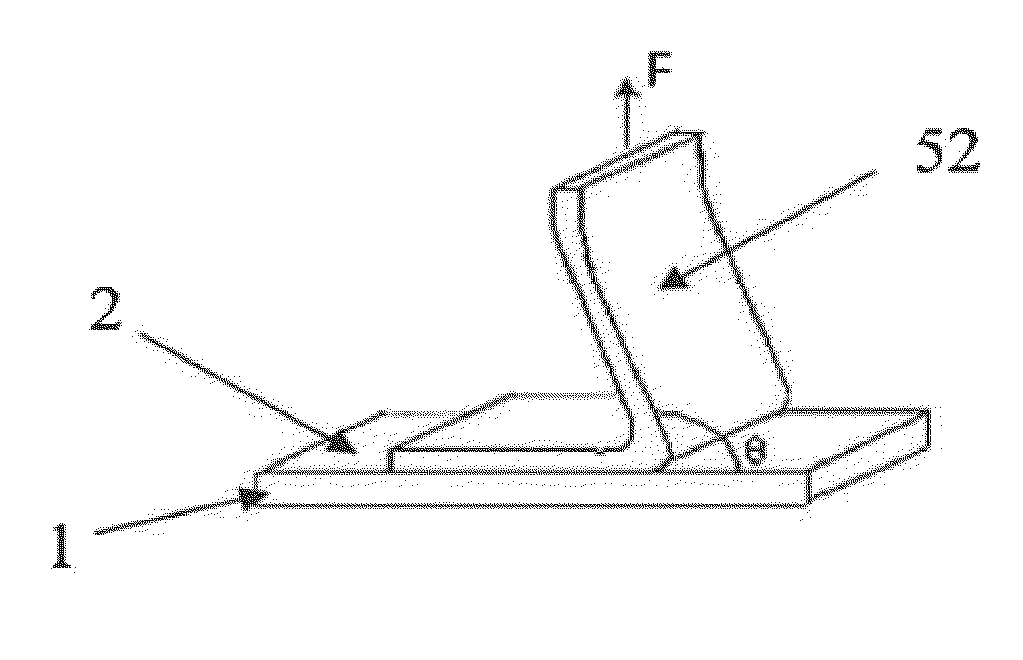
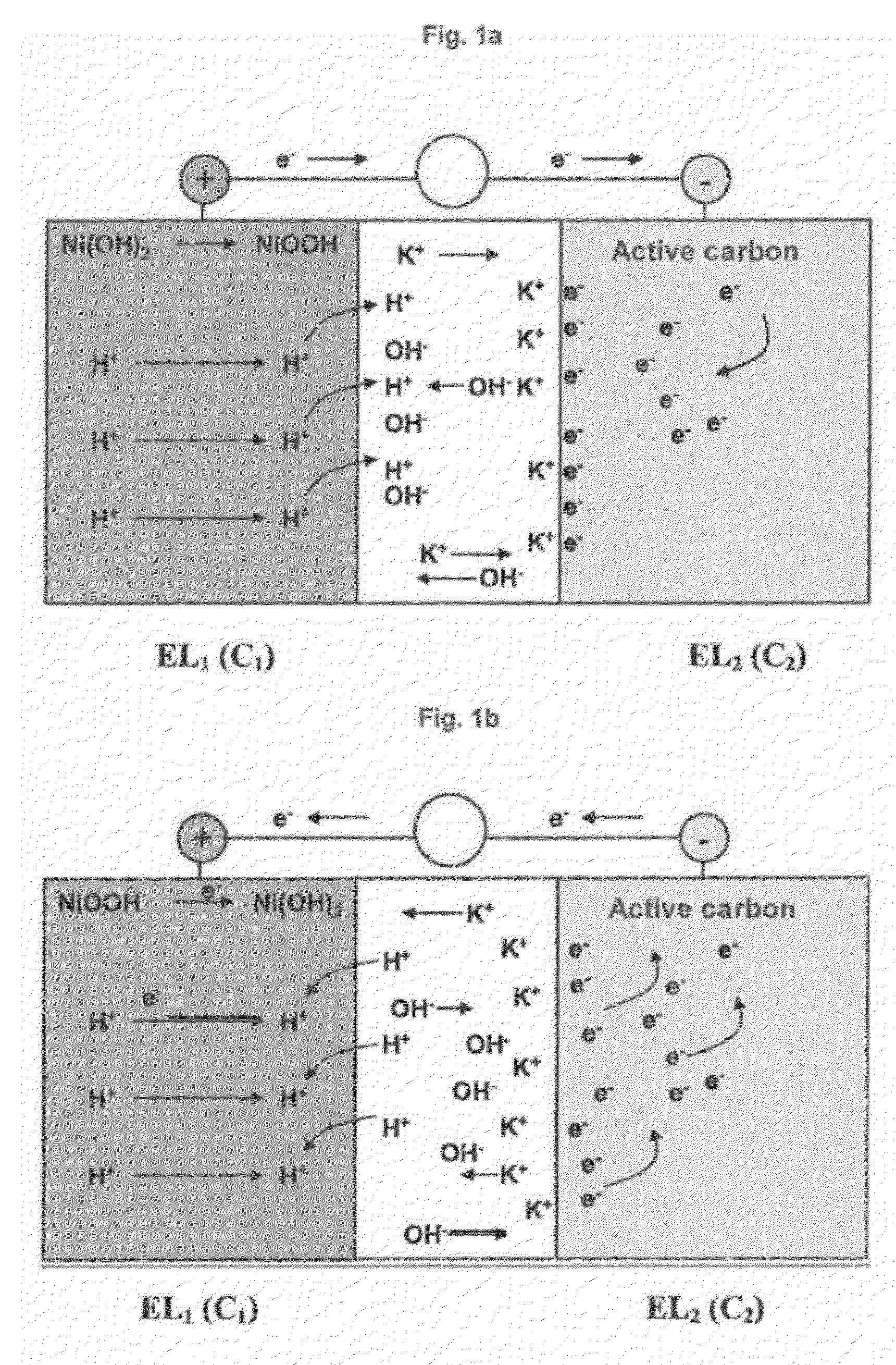
Negative electrode for asymmetric supercapacitor having a positive electrode based on nickel hydroxide and an alkaline electrolyte and method for making same
InactiveUS20120300367A1Good voluminalGood mass capacitanceNon-insulated conductorsElectrolytic capacitorsActivated carbonSupercapacitor
The object of the invention is an electrode comprising a continuous current collector (1) wherein 50% or more of the surface of said collector has a roughness Ra1 comprised between 0.8 and 15 μm, measured for a base length comprised between 2 and 200 μm, said collector being coated with a mixture (2) comprising active carbon having a specific surface area greater than or equal to 500 m2 / g and a binder comprising a mixture of at least one cellulosic compound and at least one styrenic copolymer. The object of the invention is also an asymmetric supercapacitor comprising at least one electrode according to the invention. The invention also relates to a method for making a carbon electrode for an asymmetric supercapacitor.
Owner:SAFT GRP SA
Secondary bismuth oxychloride/nickel hydroxide alkaline battery and preparation method thereof
InactiveCN106450507AGood electrical propertiesLarge capacityFinal product manufactureCell electrodesSolventIon
The invention discloses a secondary bismuth oxychloride / nickel hydroxide alkaline battery and a preparation method thereof. Bismuth oxychloride serves as negative electrode materials, nickel hydroxide serves as positive electrode materials, and alkaline solution serves as electrolyte solution. A preparation method of the bismuth oxychloride includes the steps: dripping deposition agents into bismuth chloride solution, and performing reaction; or hydrolyzing bismuth nitrate or bismuth sulfate to form supernatant solution, and dripping chloride ion solution into the supernatant solution according to a certain mole ratio of bismuth and chlorine; or mixing bismuth materials and solution to form supernatant solution, and dripping hydrochloric acid solution into the supernatant solution according to a certain mole ratio of the bismuth and the chlorine; controlling terminal PH (potential of hydrogen) to be 1-5, and preparing the bismuth oxychloride materials formed by nano sheets. The thickness of each bismuth oxychloride material ranges from 5nm to 100nm. Specific capacity of the battery prepared by the method is 298mAh / g under 1A / g of current density. The battery is a novel reversible secondary chemical power supply with wide application prospects, large in electric capacity, excellent in electrochemical performance and environmentally friendly.
Owner:XIANGTAN UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com