Self-collecting supercapacitor electrode material and preparing method thereof
A technology of supercapacitors and electrode materials, applied in hybrid capacitor electrodes, hybrid/electric double layer capacitor manufacturing, nanotechnology, etc., can solve the problem of direct use of b-nickel hydroxide supercapacitor electrode materials, use of chemical reagents, and easy pollution and other problems, to achieve the effect of easy mass preparation and industrial production, low cost, and zero pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
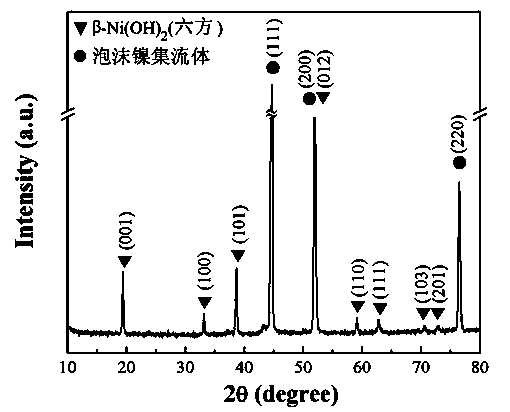
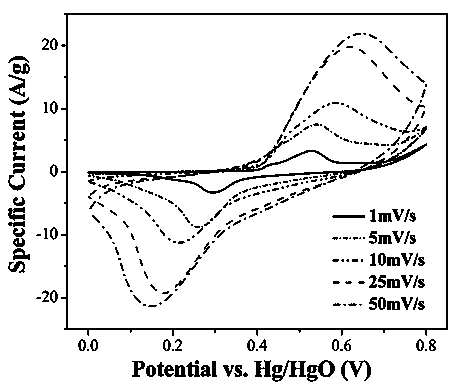
[0019] Divide the area to 2cm 2 The cleaning foam nickel is immersed in a 60cm 3 , 15% (mass percent concentration) hydrogen peroxide solution reaction kettle, seal the reaction kettle and place it in an oven at 180°C for 24 hours, then cool to room temperature, take out the foamed nickel, rinse the foamed nickel with pure water, and then Put it in a vacuum oven at 60°C and dry it for 5 hours to obtain the current collector electrode material. from figure 1 with figure 2 The results show that the self-collecting electrode material is composed of nickel foam current collector and b-nickel hydroxide (hexagonal structure) hexagonal nanosheets grown in situ on its surface. from Figure 3~6 The performance test results show that the self-collecting electrode material has a small electrode impedance and a high electron transfer rate, a large specific volume, good cycle stability, and a specific volume greater than 1000 F / g at a scan rate of 5mV / s. The specific capacity retenti...
Embodiment 2
[0021] Divide the area to 1cm 2 The cleaning foam nickel is immersed in a 50cm 3 , 10% (mass percent concentration) hydrogen peroxide solution in the reaction kettle, seal the reaction kettle and place it in an oven at 200 ° C for 20 hours, then cool to room temperature, take out the foamed nickel, rinse the foamed nickel with pure water, and then Dry it in a vacuum oven at 70°C for 4 hours to obtain a self-collecting electrode composed of a nickel foam current collector and b-nickel hydroxide (in a hexagonal structure) hexagonal nanosheets grown in situ on its surface Material. The self-collecting electrode material has very small electrode impedance and high electron transfer rate, large specific volume and good cycle stability. The specific capacity retention after 2000 cycles at the scan rate is over 95%.
Embodiment 3
[0023] Divide the area to 3cm 2 The cleaning foam nickel is immersed in a 30cm 3 , 5% (mass percent concentration) hydrogen peroxide solution in a reaction kettle, seal the reaction kettle and place it in an oven at 240°C for 10 hours, then cool to room temperature, take out the foamed nickel, rinse the foamed nickel with pure water, and then Dry in a vacuum oven at 80°C for 3 hours to obtain a self-collecting electrode composed of a nickel foam current collector and b-nickel hydroxide (in a hexagonal structure) hexagonal nanosheets grown in situ on its surface Material. The self-collecting electrode material has very small electrode impedance and high electron transfer rate, large specific volume and good cycle stability. The specific capacity retention after 2000 cycles at the scan rate is over 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com