Ferronickel hydroxide/reduction-oxidation graphene electrochemical oxygen evolution catalyst with nickel foam as carrier and preparation method of ferronickel hydroxide/reduction-oxidation graphene electrochemical oxygen evolution catalyst
An electrochemical technology of hydroxide and olefin, applied in the field of electrocatalytic materials, can solve the problems of increasing catalyst resistance, complex preparation process, unfavorable catalytic performance, etc., achieve simple preparation method, ensure stability, and avoid electrode preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
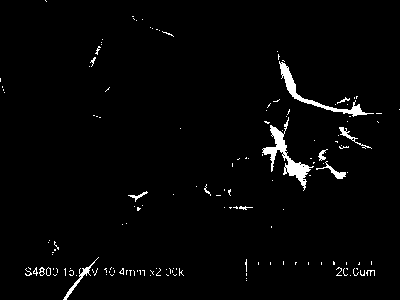
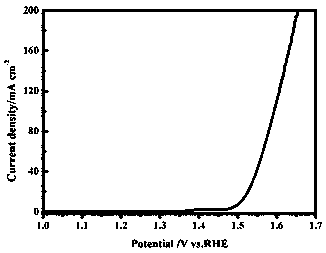
[0023] Preparation of nickel iron hydroxide / reduced graphene oxide electrochemical oxygen evolution catalyst supported by nickel foam
[0024] Nickel foam was treated with acetone, ethanol, and deionized water ultrasonically for 30 minutes to remove impurities on the surface of nickel foam. o C, dry under the condition of 10h for use; then weigh 1mmol nickel nitrate, 8mmol urea and dissolve in 46ml deionized water, stir at room temperature to obtain a uniform mixed solution; then add 4ml to the mixed solution, the concentration is 5mg L -1 Graphene oxide solution was ultrasonically treated in an ultrasonic pool for 30 minutes to obtain a uniformly dispersed mixed solution; the clean nickel foam and the mixed solution were transferred to a 100ml hydrothermal kettle and sealed at 200 o Under the condition of C, react for 12h; Clean the foam nickel after reaction with ethanol and deionized water, place in 60 o Dry in an oven at C; then soak in 10mmol L -1 8h in ferric nitrate, ...
Embodiment 2
[0026] Preparation of nickel hydroxide / reduced graphene oxide electrochemical oxygen evolution catalyst supported by nickel foam
[0027] Nickel foam was treated with acetone, ethanol, and deionized water for ultrasonic treatment for 15 minutes to remove impurities on the surface of nickel foam, 60 o C, dry under the condition of 10h for use; then weigh 0.5mmol nickel chloride, 2.5mmol urea and dissolve in 48ml deionized water, stir at room temperature to obtain a uniform mixed solution;
[0028] Then add 2 ml to the mixture, the concentration is 5 mg L -1 Graphene oxide solution was ultrasonically treated in an ultrasonic pool for 30 minutes to obtain a uniformly dispersed mixed solution; the clean nickel foam and the mixed solution were transferred to a 100ml hydrothermal kettle and sealed, and reacted for 24h at 120°C; Ethanol and deionized water cleaned the foamed nickel after the reaction, and placed it at 80 o Dried in the oven of C; do not do the impregnation treatmen...
Embodiment 3
[0030] Preparation of nickel iron hydroxide / reduced graphene oxide electrochemical oxygen evolution catalyst supported by nickel foam
[0031] Nickel foam was treated with acetone, ethanol, and deionized water ultrasonically for 30 minutes to remove impurities on the surface of nickel foam, 70 o C, dry under the condition of 10h for use; then weigh 0.25mmol nickel citrate, 2mmol urea and dissolve in 27ml deionized water, stir at room temperature to obtain a uniform mixed solution;
[0032] Then add 3ml to the mixture, the concentration is 5mg L -1 Graphene oxide solution was ultrasonically treated in an ultrasonic pool for 30 minutes to obtain a uniformly dispersed mixed solution; the clean nickel foam and the mixed solution were transferred to a 50ml hydrothermal kettle and sealed, and reacted for 15h at 120°C; Ethanol and deionized water cleaned the foamed nickel after the reaction, and placed it at 70 o Dry in an oven at C; then soak in 5mmol L -1 ferric citrate for 24 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com