Multi-level structure alpha type nickel hydroxide prepared by microwave auxiliary and method thereof
A microwave-assisted nickel hydroxide technology, which is applied in the direction of nickel oxide/nickel hydroxide, nanostructure manufacturing, and manipulation of single atoms, can solve the problems of long reaction time and achieve fast reaction time, simple preparation process, and short preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: α-type nickel hydroxide nano petals
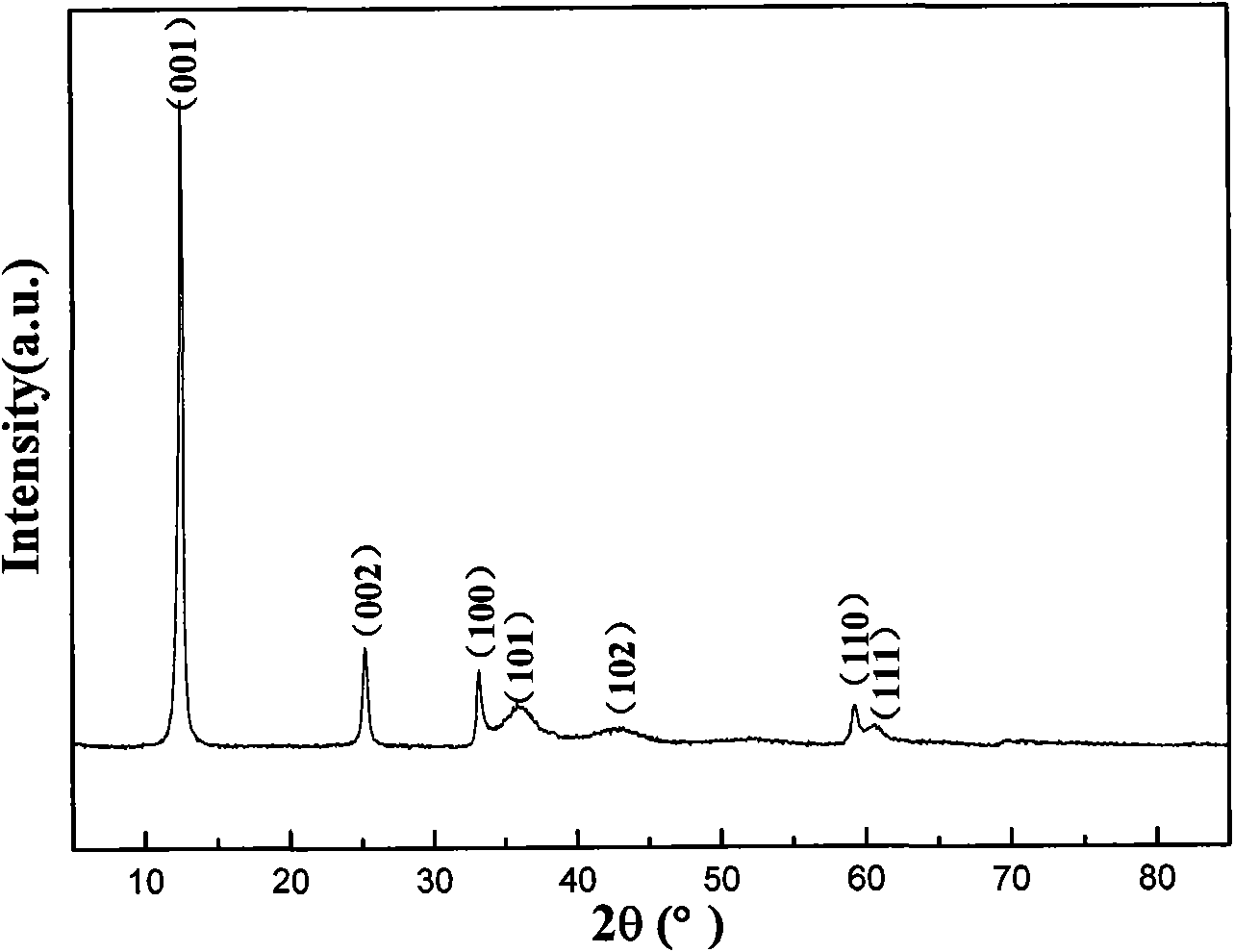
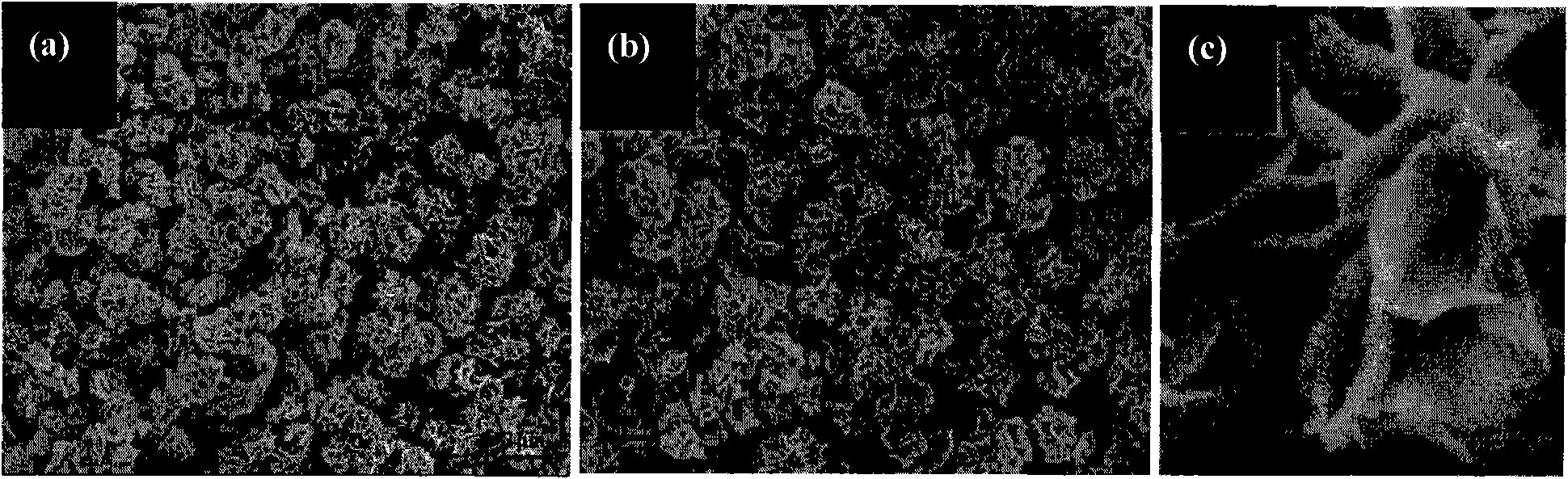
[0028] 0.581 g of nickel nitrate and 0.119 g of urea were dissolved in a mixed solution of 10 ml of ultrapure water and 10 ml of ethanol, and magnetically stirred to obtain a uniformly dispersed solution. The solution was transferred to a 30 ml quartz reaction vial and sealed. Put the reaction vial into a microwave reaction device, and react rapidly at 120°C for 1 minute. The reaction flask was quickly cooled to room temperature under the blowing of compressed air, and the product was separated by centrifugation. The obtained product was washed repeatedly with ultrapure water and absolute ethanol, and dried in air at 80°C. figure 2 It is clearly seen from the scanning electron microscope photo that the obtained nano petals are flakes, and a large number of petals are stacked together irregularly.
Embodiment 2
[0029] Embodiment 2: α-type nickel hydroxide nanoflowers
[0030] 0.581 g of nickel nitrate and 0.119 g of urea were dissolved in a mixed solution of 10 ml of ultrapure water and 10 ml of ethanol, and magnetically stirred to obtain a uniformly dispersed solution. The solution was transferred to a 30 ml quartz reaction vial and sealed. Put the reaction bottle into a microwave reaction device, and react rapidly at 100°C (or 180°C) for 30 minutes. The reaction flask was quickly cooled to room temperature under the blowing of compressed air, and the product was separated by centrifugation. The obtained product was washed repeatedly with ultrapure water and absolute ethanol, and dried in air at 80°C. image 3 a and 3b are scanning electron micrographs of samples obtained at 100 and 180 °C, respectively, and the generation of nanoflower-like morphology can be seen. Each flower is composed of more than a dozen nano-petals connected to each other, and the thickness of the nano-petal...
Embodiment 3
[0031] Embodiment 3: α-type nickel hydroxide nanoflowers
[0032] 0.474g of nickel chloride and 0.119g of urea were dissolved in a mixed solution of 10 ml of ultrapure water and 10 ml of ethanol, and magnetically stirred to obtain a uniformly dispersed solution. The solution was transferred to a 30 ml quartz reaction vial and sealed. Put the reaction bottle into a microwave reaction device, and react rapidly at 120°C for 30 minutes. The reaction flask was quickly cooled to room temperature under the blowing of compressed air, and the product was separated by centrifugation. The obtained product was washed repeatedly with ultrapure water and absolute ethanol, and dried in air at 80°C. Figure 4 From the corresponding scanning electron micrographs, it can be seen that its appearance is still flower-like, but the thickness of the nano-petals that make up the flower is relatively thin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com