Recombinant TAT-XIAP fusion protein
A fusion protein and protein transduction technology, applied in the field of fusion proteins, can solve the problems of gene expression regulation, cumbersome operation, and difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1TAT-XIAP fusion protein
[0036] 1. Construction of pTAT-XIAP fusion protein prokaryotic expression vector
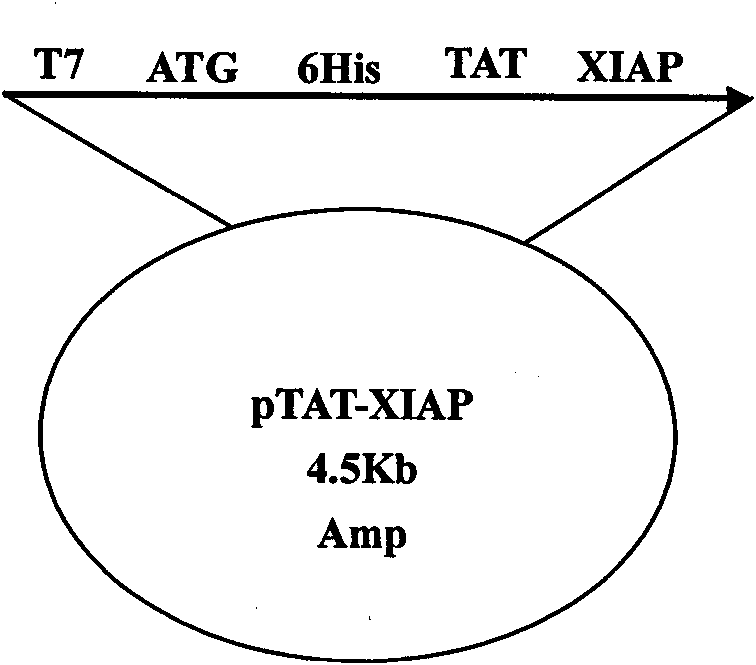
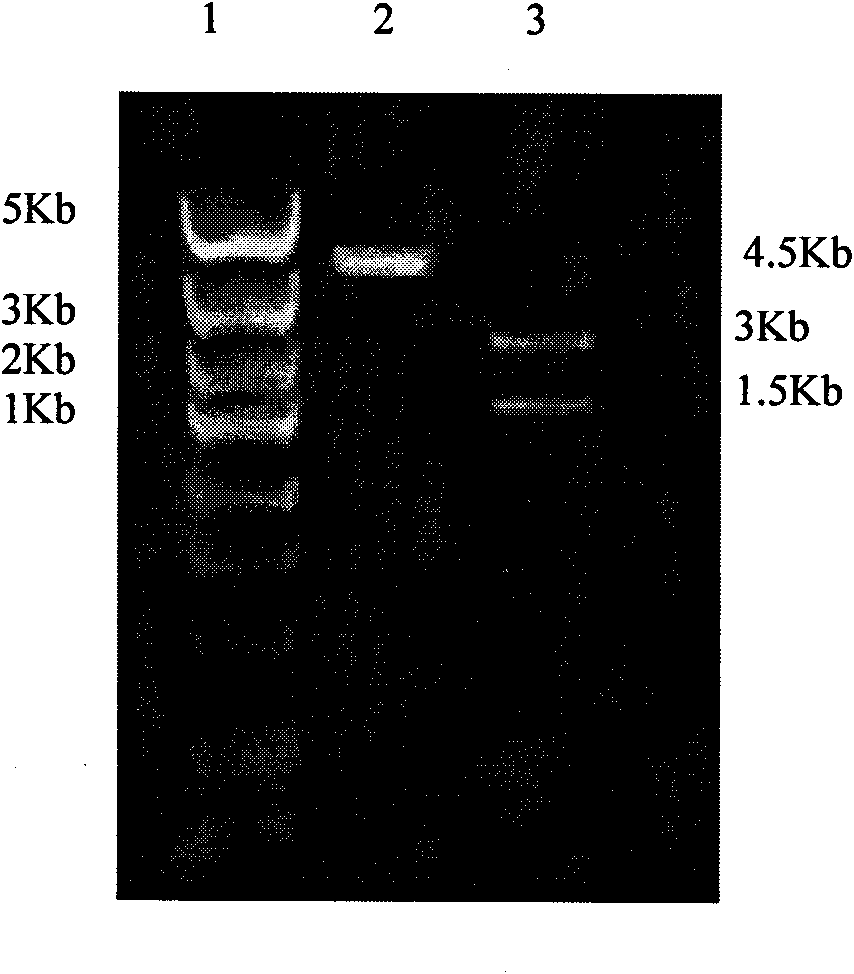
[0037] According to the DNA sequence of rat XIAP provided by the NCBI gene bank, it was optimized according to the preferred codons of Escherichia coli, and the gene sequence was synthesized by Shanghai Sangon Biotechnology Service Co., Ltd., with NcoI and XhoI restriction sites at both ends. The pTAT-HA plasmid was double digested with NcoI and XhoI enzymes, and the XIAP gene was inserted between the NcoI and XhoI restriction sites to construct the pTAT-XIAP fusion protein prokaryotic expression vector. The plasmid vector schematic diagram is as follows figure 1 As indicated, the pET-32a-XIAP expression vector was additionally constructed to produce XIAP as a control.
[0038] 2. Transformation of pTAT-XIAP recombinant plasmid in Escherichia coli
[0039] Take 100 μl of DH5α competent cells in an ice water bath, add 10 μl of TAT-XI...
Embodiment 2
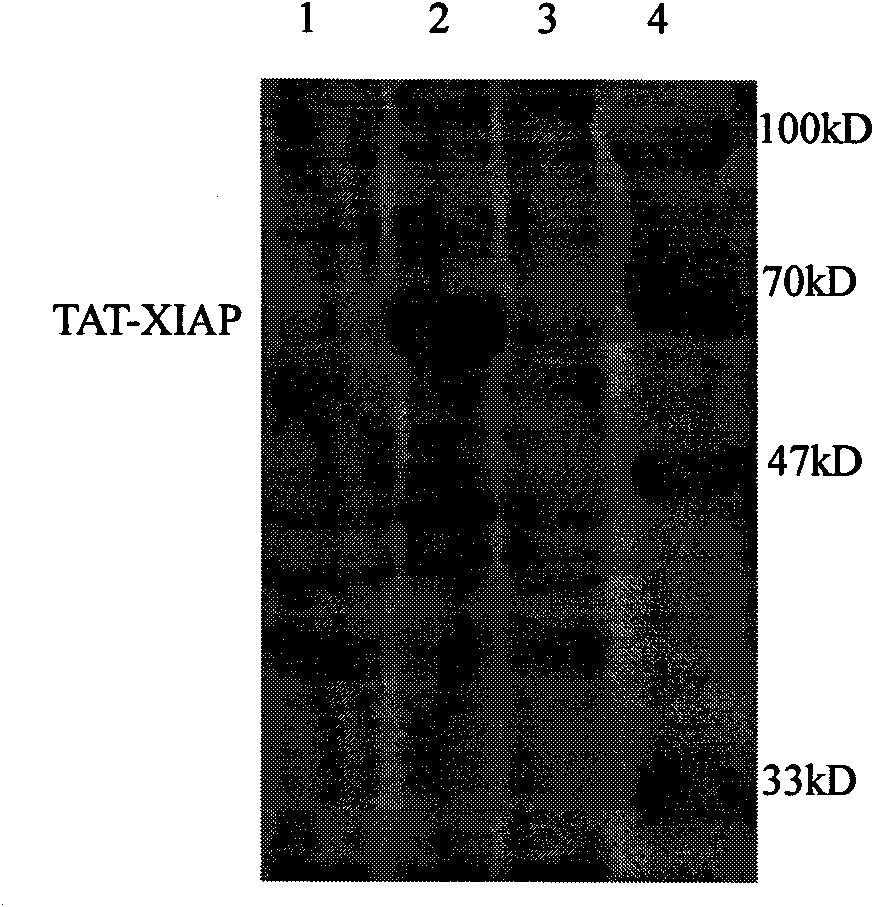
[0048] Example 2 Detection of TAT-XIAP fusion protein's ability to penetrate nerve cell membranes
[0049] Take newborn SD rats, take brains under aseptic conditions, digest with 0.125% trypsin (37°C, 30min) and disperse, then dilute to 5×10 with DMEM medium 5 The cell liquid of 1 cell / ml density is inoculated in the petri dish that is coated with polylysine, adds the TAT-XIAP fusion protein (test group) after the purification of 1~2 μ l embodiment 1 in culture liquid, mixes gently, After culturing for 6-12 hours, make cell slides, detect XIAP by immunocytochemistry (SP method), and observe the ability of the fusion protein to pass through the cell membrane.
[0050] The results showed that as the concentration of the fusion protein increased, the positive staining increased, but no obvious positive staining was seen in the control group treated with XIAP or PBS, indicating that the fusion protein containing the TAT protein transduction domain had entered the brain cells.
Embodiment 3
[0051] Example 3 Detection of the ability of TAT-XIAP fusion protein to cross the blood-brain barrier
[0052] SD rats were injected into the tail vein of 10-20 μl of the purified TAT-XIAP fusion protein (test group), XIAP (negative control group) and PBS (blank control group) in Example 1 respectively for 6-12 hours, and immunohistochemistry (SP method) was performed. ) to detect XIAP in brain tissue slices, and to observe the ability of the fusion protein to pass through the blood-brain barrier.
[0053] The results showed that the positive staining in the test group increased with the increase of the concentration of TAT-XIAP fusion protein, but no obvious positive staining was seen in the control group. It shows that the fusion protein containing the TAT domain enters the brain cells through the blood-brain barrier.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com