Myrcenyl plasticizer and preparation method thereof
A technology of plasticizer and myrcene, which is applied in the field of plasticizers that replace DOP and its preparation, can solve problems such as high price and inferior performance to DOP, and achieve the effects of saving energy, simple process and expanding added value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The preparation method of described myrcene-based plasticizer, more specific steps are as follows:
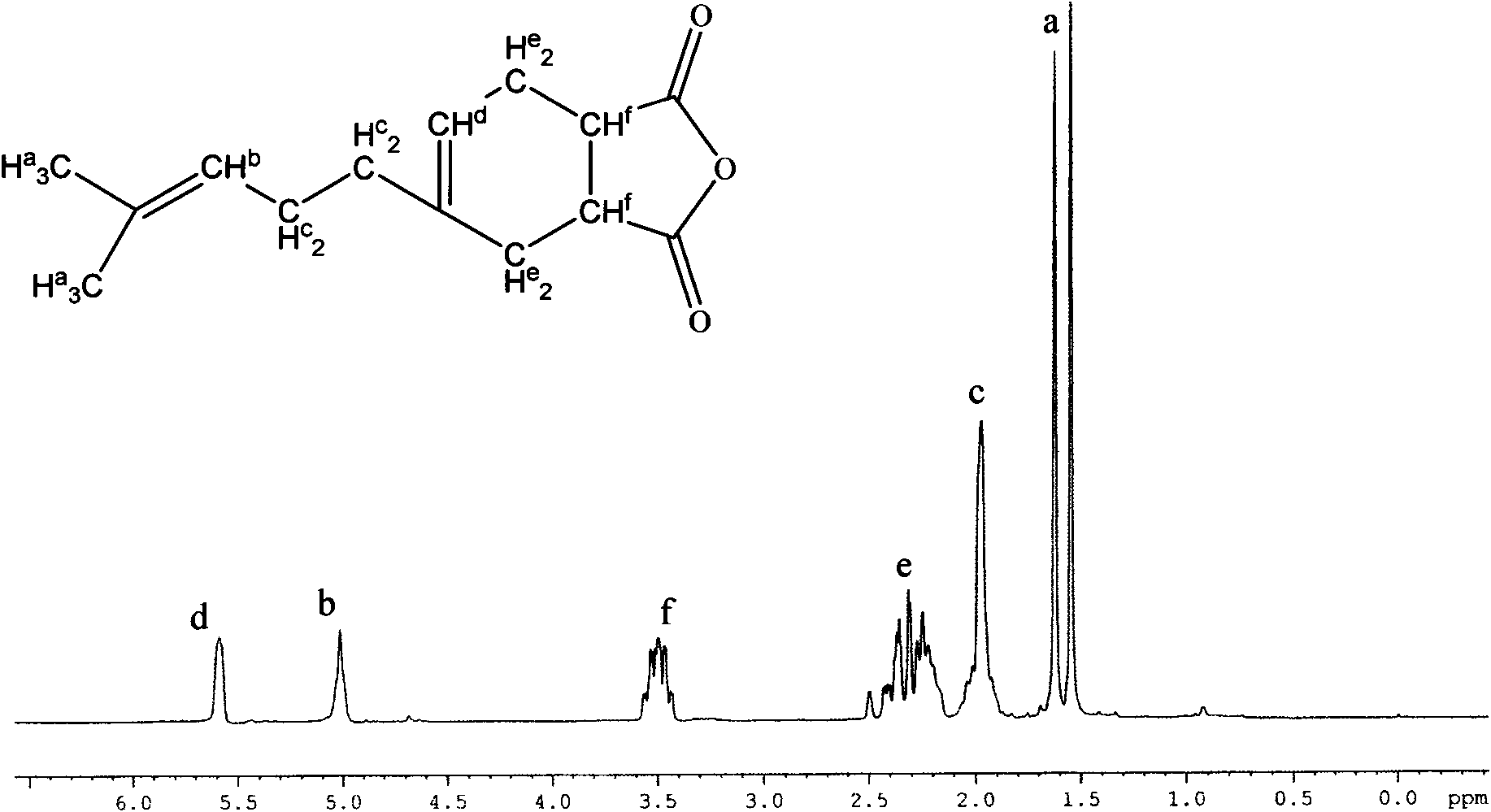
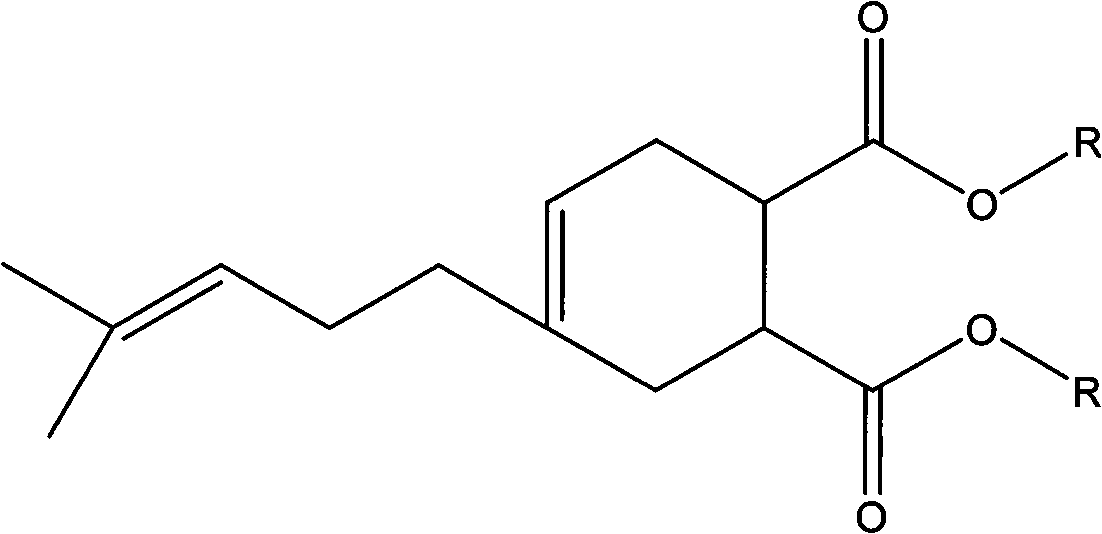
[0043] (1) Under the conditions of 30-80°C and no catalyst, carry out Diels-Alder reaction of equimolar amounts of myrcene and unsaturated anhydride for 3-7 hours to obtain the intermediate product 4-(4-methyl-3-pentane Alkenyl)-4-cyclohexene-1,2-acid (anhydride) (abbreviated as MYM).
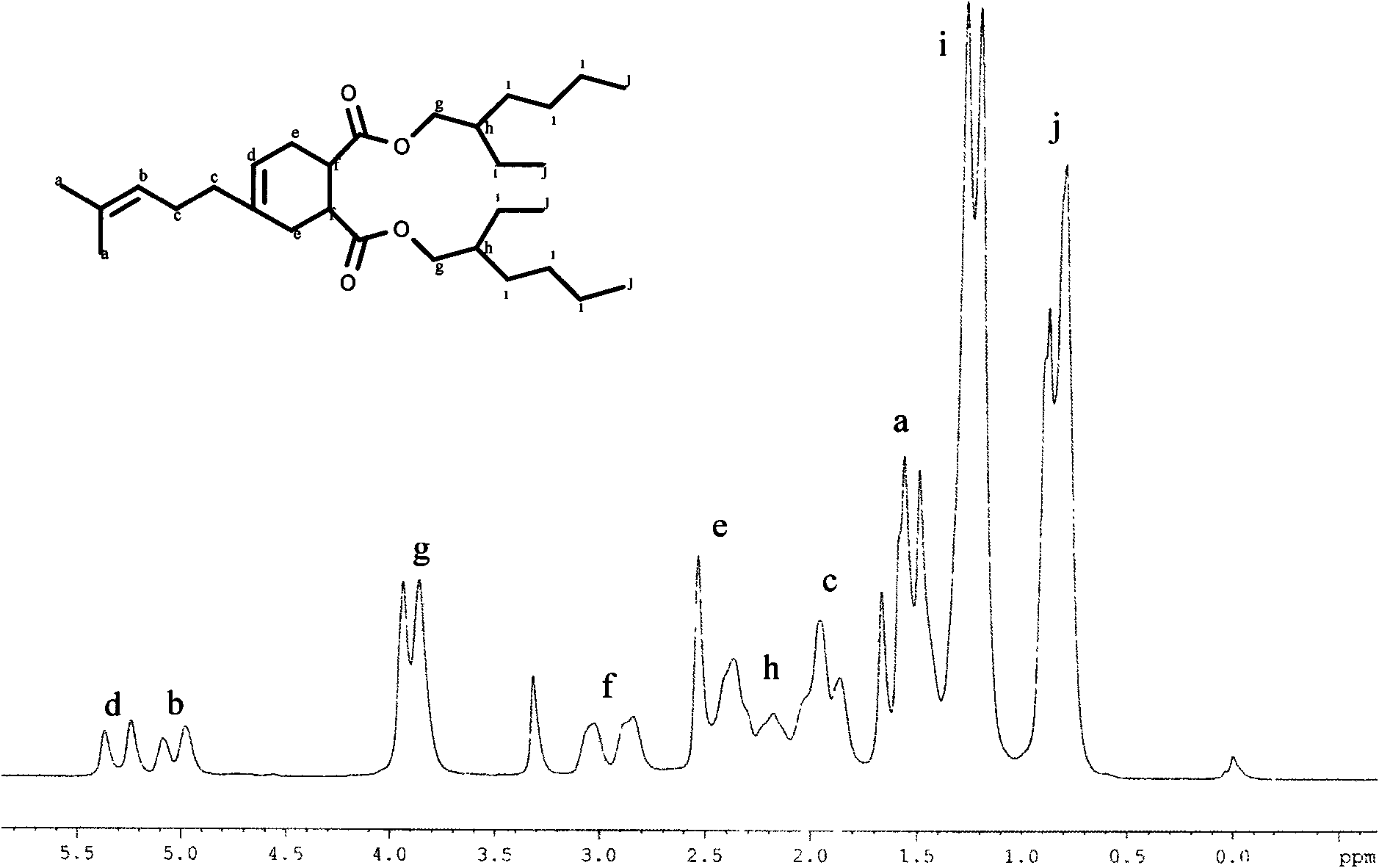
[0044] (2) Use protonic acid or Lewis acid as a catalyst, the dosage is 0.5% to 3% of the molar weight of MYM, add a water-carrying agent of 5% to 25% of the total mass, and carry out esterification reaction between MYM and alcohol at a temperature of reflux After 0.5-5 hours, the target plasticizer was obtained.
[0045] The unsaturated acid or acid anhydride is selected from maleic anhydride, maleic acid, fumaric acid and other non-phthalic anhydrides or acids.
Embodiment 1
[0047] (1) In a heating jacket, a stirring device, a thermometer, a dropping funnel and a reflux condenser (the condenser is connected to a built-in CaCl 2 In the 500mL four-neck flask of drying tube), add 0.8mol maleic anhydride and heat it to melt. Start the stirrer, maintain the temperature at 50°C to 60°C, drop an equimole of myrcene from the dropping funnel, control the dropwise addition within 0.5h, and raise the temperature to 70°C for 4h after the dropwise addition. After the reaction is finished, vacuum distillation is used to collect the fraction at 180° C. / 1.05 kPa (absolute pressure), which is the intermediate product MYM.
[0048] (2) Take 10g SnCl 2 In a 150mL beaker, add 45g of distilled water, ultrasonic vibration to SnCl 2 Dissolve, and then dropwise add ammonia water to the beaker to pH = 7, let it stand for 60 minutes, separate layers, and remove the SnO layer for later use.
[0049] (3) In a 250mL three-neck flask equipped with a heating mantle, a stirri...
Embodiment 2
[0051] (1) In a heating jacket, a stirring device, a thermometer, a dropping funnel and a reflux condenser (the condenser is connected to a built-in CaCl 2 In the 500mL four-neck flask of drying tube), add 0.8mol maleic acid. Start the stirrer, add equimolar myrcene dropwise from the dropping funnel, control the dropwise addition to be completed within 0.5h, and raise the temperature to 70°C for 4h reaction after the dropwise addition. After the reaction is finished, vacuum distillation is used to collect the fraction at 180° C. / 1.05 kPa (absolute pressure), which is the intermediate product MYM.
[0052](2) In a 250mL three-neck flask equipped with a heating mantle, a stirring device, a thermometer, and a water separator (the water separator is connected to a reflux condenser), add 0.1mol MYM and 0.4mol isooctyl alcohol, and simultaneously add 45g of toluene and 0.2 g Composite catalyst SnO-ZnO, MYM and isooctyl alcohol carry out esterification reaction at reflux temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com