Methacycline derivative
A technology of substances and compounds, applied in the field of derivatives of metacycline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0004] Embodiment 1: the preparation of formula 1 compound
[0005] (1) Preparation of 9-nitrometacycline sulfate A certain amount of metacycline hydrochloride (1kg, purchased from Wuhan Yuancheng Chemical Co., Ltd.) was added to 96%-98% concentrated sulfuric acid (4kg), The temperature is kept below 10°C. After cooling the solution to below 7°C, concentrated nitric acid (concentration greater than 90%, 0.2kg) was added dropwise, and the reaction mixture was stirred while the temperature was less than 10°C. Pour the reaction mixture into a mixture of isopropanol (25L) and heptane (4L) at a temperature of 0-5°C, keep the temperature below 40°C and stir the suspension until the suspension is cooled to below 25°C , centrifuge 9-nitrometacycline sulfate and wash with a mixture of isopropanol and methanol. Product 1 was obtained by drying at 45°C.
[0006] (2) The obtained product 1 was dissolved in a mixture of methanol (7 L) and purified water (0.1 L). 10% palladium-carbon (0...
Embodiment 2
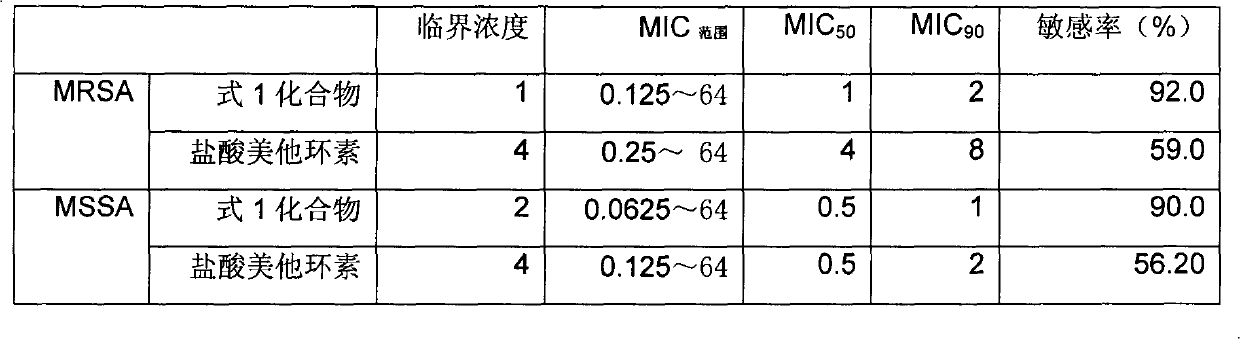
[0010] Embodiment 2: the antibacterial activity research of formula 1 compound
[0011] 2.1 Materials and methods
[0012] Antibacterial drug: compound of formula 1
[0013] Control drug: metacycline hydrochloride was purchased from Wuhan Yuancheng Chemical Co., Ltd.
[0014] Tested strains: 101 strains of MRSA and 105 strains of MSSA were donated by the School of Pharmacy, Sun Yat-sen University, and determined according to the NCCLS standard after identification.
[0015] Culture medium: purchased from BioMérieux, France, batch number: 811813401.
[0016] Method: The minimum inhibitory concentration (MIC) of the compound of formula 1 and metacycline hydrochloride against all strains was determined by double agar dilution method. That is, first dilute the two antibiotics to 12 concentrations with sterile phosphate buffer solution of different concentrations and pH, and add 10ml of each concentration of the drug solution to the M-H agar that has been melted and cooled to ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com