Method for removing and recycling hexavalent chromium ions by using chitosan-iron complex
A technology of hexavalent chromium ions and iron complexes, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of cumbersome preparation, new material removal efficiency needs to be improved, and inconvenient operation. Simplicity, improved adsorption performance, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
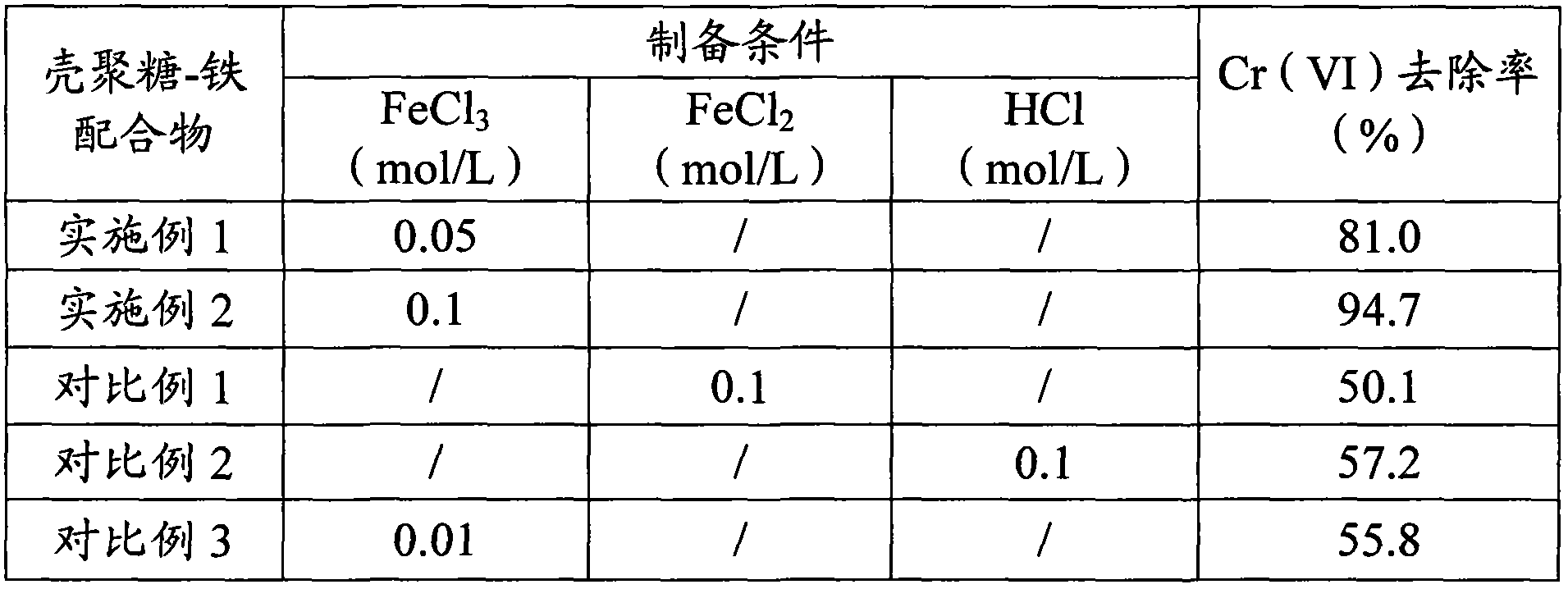
[0030] Prepare 0.05mol / L FeCl 3 Solution 50mL (0.0025mol), slowly add chitosan monomer 2.67g (molecular weight is 1,000,000, deacetylation degree is 91%), make chitosan monomer and Fe 3+ The molar ratio of the chitosan-iron complex is 6:1, keep stirring for 2 hours to make the two fully react, then slowly add 50mL of ethanol and stir slowly at the same time, after the chitosan-iron complex precipitation is gradually formed, centrifuge and wash with absolute ethanol to unreacted Fe 3+ , then add 25% glutaraldehyde 5mL, cross-linking reaction for 2h, and finally use absolute ethanol to wash glutaraldehyde again, centrifuge, dry at 80°C, grind finely, and prepare reduction-adsorption bifunctional chitosan-iron Complexes.
Embodiment 2
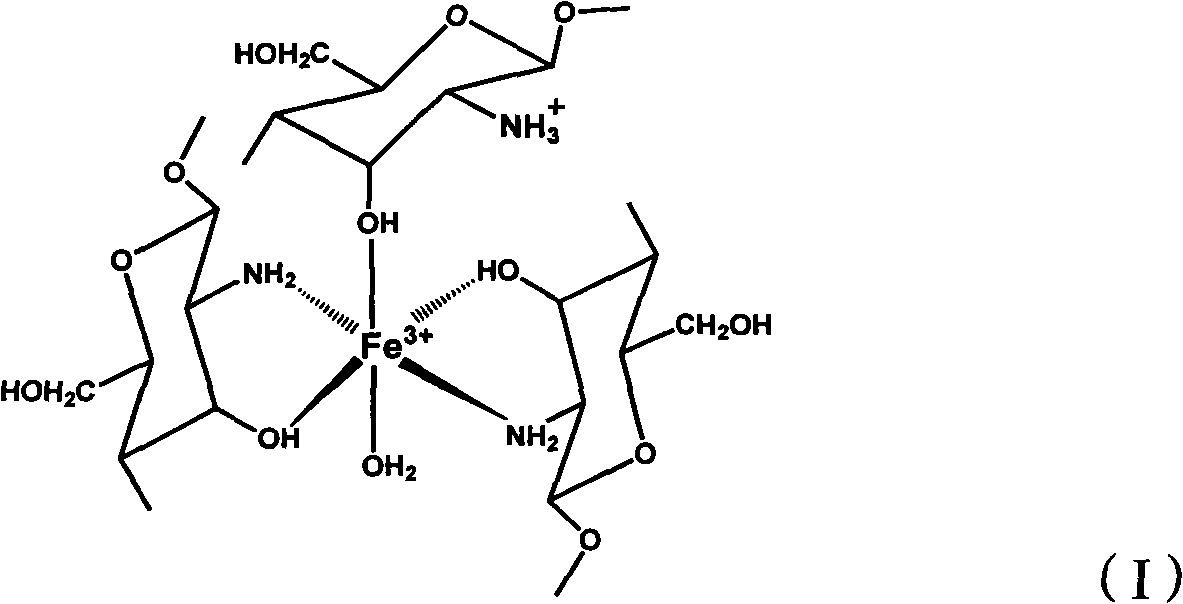
[0032] Prepare 0.1mol / L FeCl 3 Solution 50mL (0.005mol), slowly add chitosan monomer 2.67g (molecular weight is 1,000,000, deacetylation degree is 91%), make chitosan monomer and Fe 3+ The molar ratio of the chitosan-iron complex is 3:1, keep stirring for 2 hours to make the two fully react, then slowly add 50mL of ethanol and stir slowly at the same time, after the chitosan-iron complex precipitation is gradually formed, centrifuge and wash with absolute ethanol to unreacted Fe 3+ , then add 25% glutaraldehyde 5mL, cross-linking reaction for 2h, finally use absolute ethanol to wash the glutaraldehyde again, centrifuge, dry at 80°C, and grind finely to obtain the reduction-adsorption bifunctional chitosan-iron complex Thing, structure is as shown in formula (I):
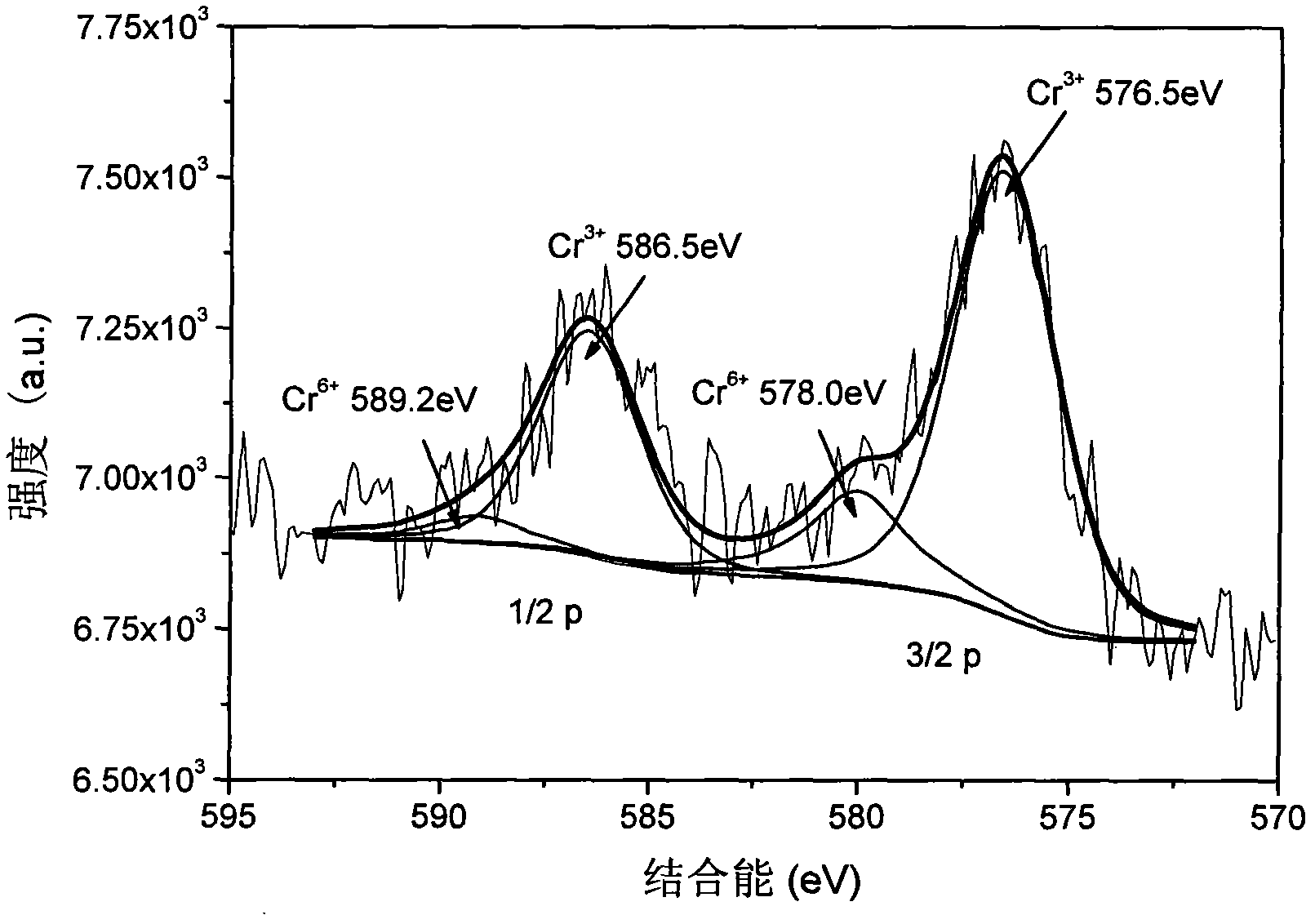
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com