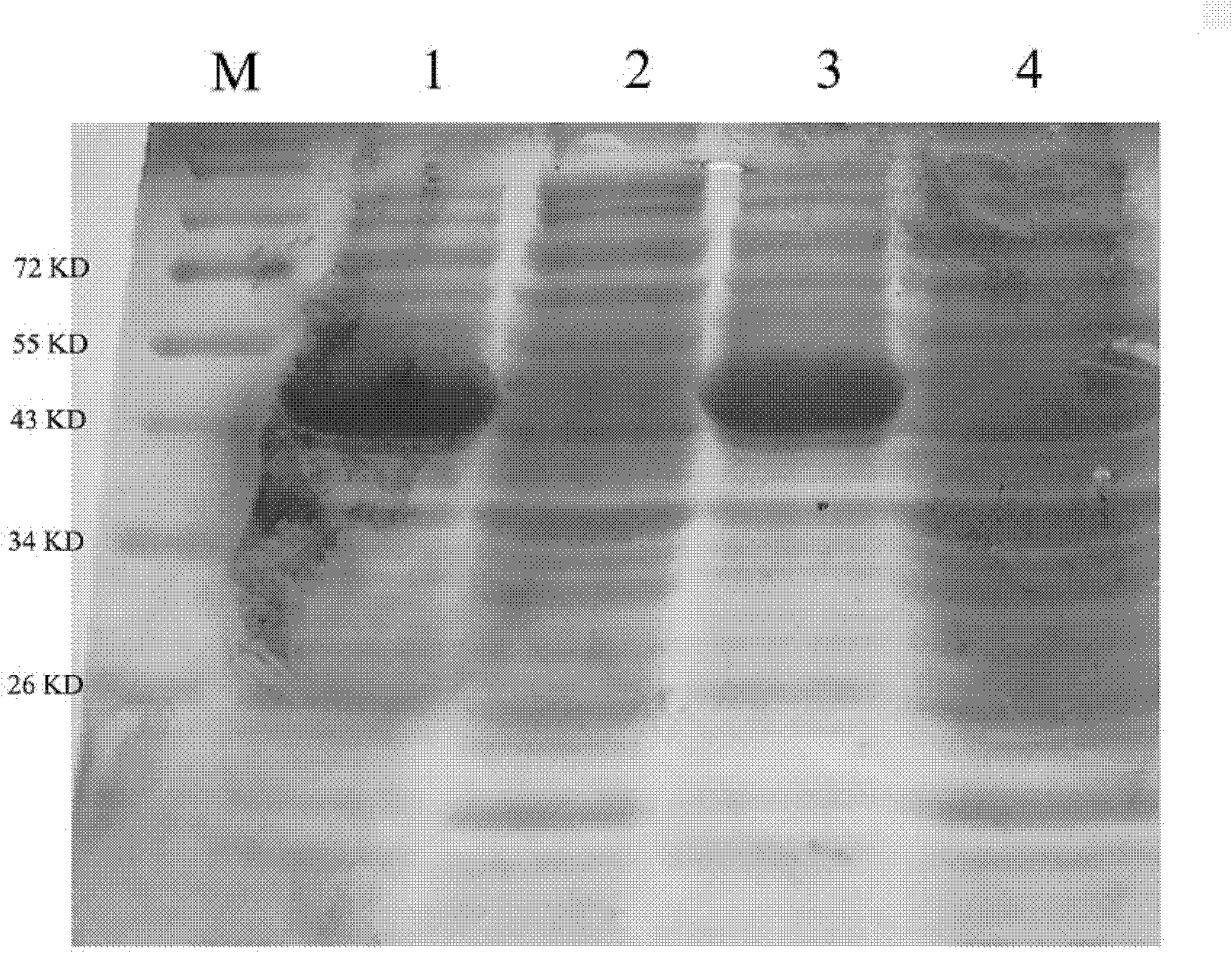Recombination fusion protein Trx-TAT-hMsrA and application thereof to aspect of nerve cell protection
A trx-tat-hmsra and fusion protein technology, applied in the field of genetic engineering, can solve the problems of poor druggability, difficult to penetrate the blood-brain barrier, difficult to enter cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
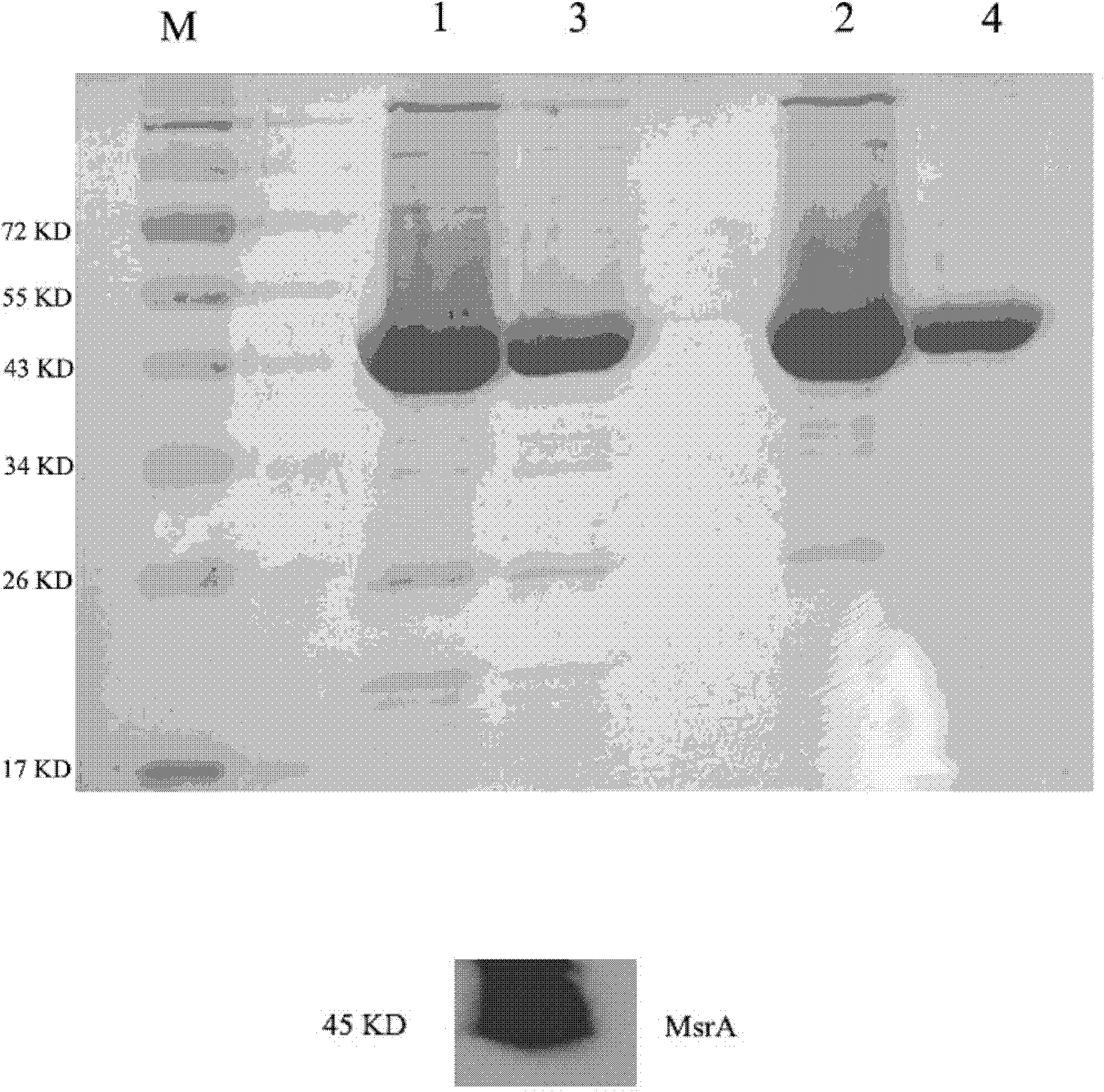
[0051] Embodiment 1 prepares fusion protein of the present invention
[0052] 1. Construction of recombinant Trx-TAT-hMsrA fusion protein expression vector
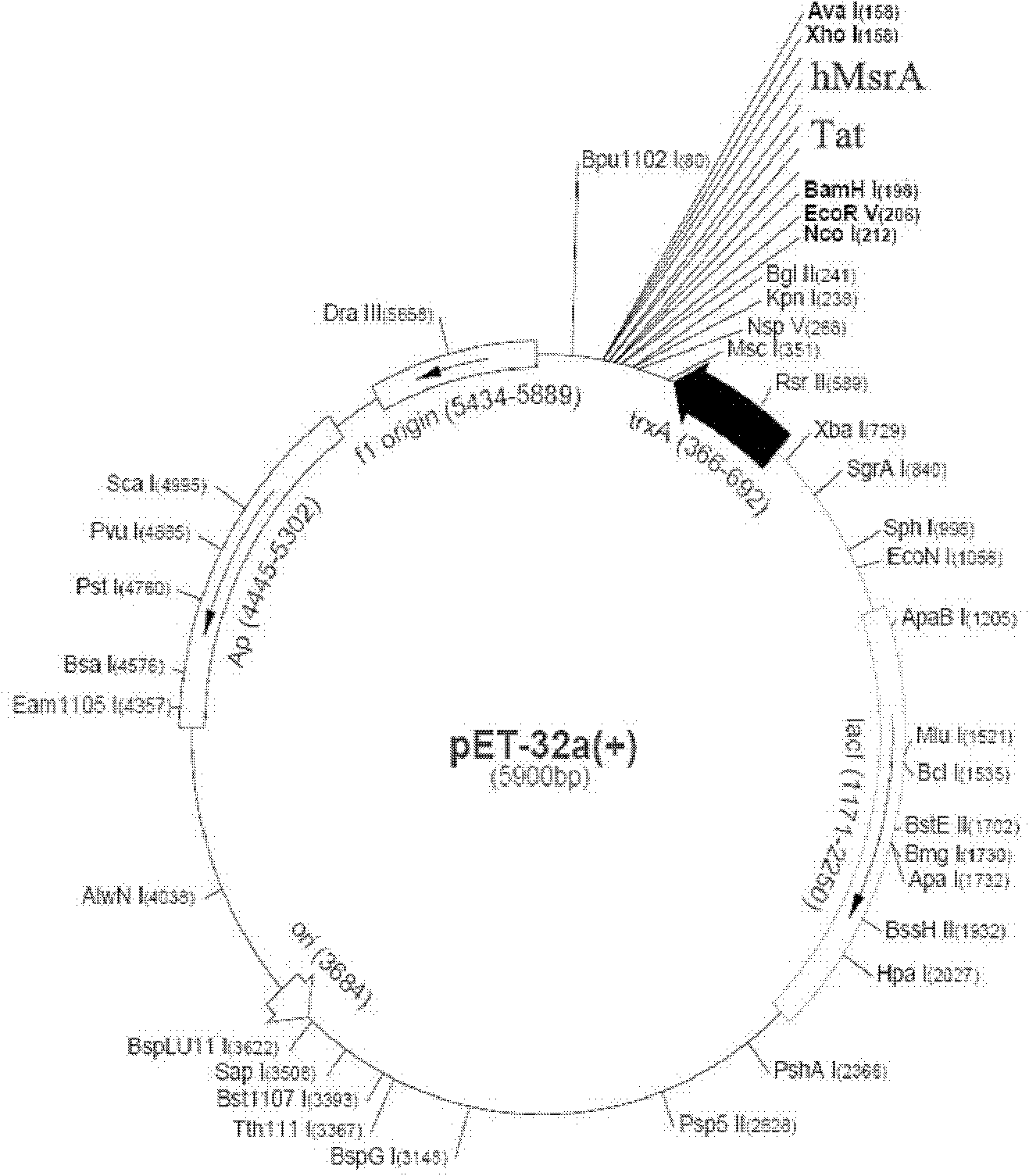
[0053] The constructed vector map is attached figure 1 .
[0054] Detailed steps of vector construction:
[0055] (1). Sequencing: The Pcmv-XL4 vector containing the hMsrA gene (Beijing Yixing Industry Technology Co., Ltd.) was sent to Beijing Aoke Biotechnology Co., Ltd. for sequencing. The sequencing results were consistent with the human-derived MsrA gene in the gene bank Consistent, indicating that the plasmid is available.
[0056] (2). Design primers: In order to extract the hMsrA gene from the Pcmv-XL4 vector and switch it to the pet32a vector, design a pair of primers with BamHI and XhoI as restriction sites, and add Upper Tat sequence, 5' end sequence: 5-CGCGGATCCGGTTACGGTCGTAAGAAACGTAGACAGCGCAGACGTGGTCGCCCTCCCATGCTCTCGGC-3;
[0057] 3' end sequence:
[0058] 5-CCGCTCGAGTTATTTTTTAATACCCACTGGGCAGGACACGC-3, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com