Preparation method of CaMoO4: Eu3+, Li+ red phosphor
A red phosphor, ca1-xmoo4 technology, applied in chemical instruments and methods, luminescent materials, etc., to achieve the effects of prolonging burning time, low energy consumption, and improving luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] According to the chemical formula Ca 1-x MoO 4 :Eu 3+ x , Li + y (x=0.18, y=0.10) Weigh the corresponding medicines.
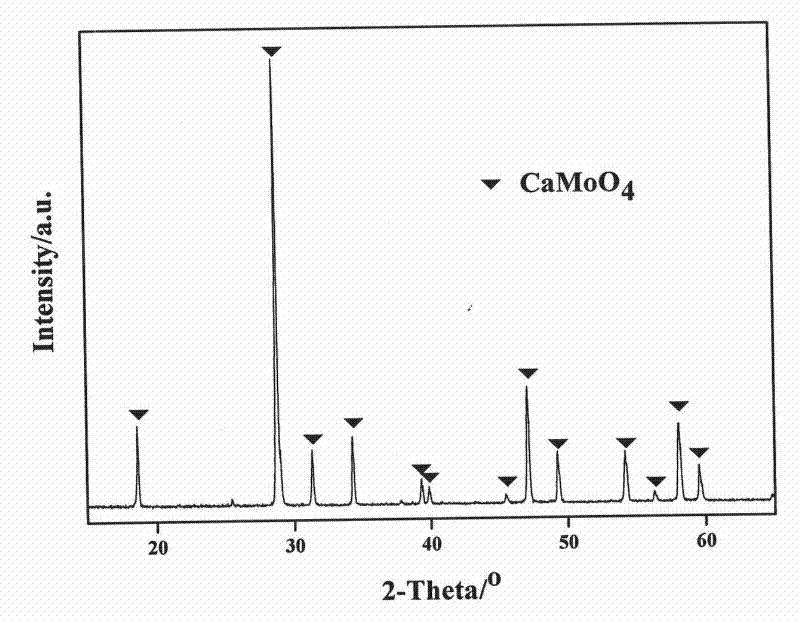
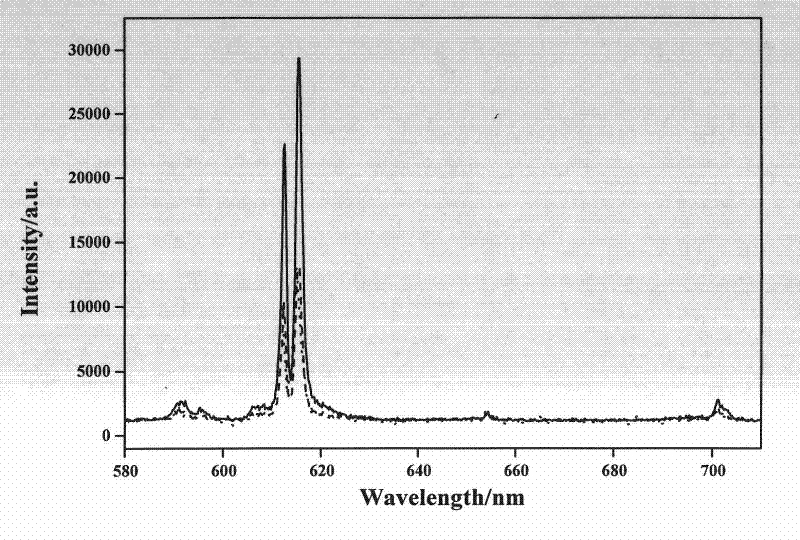
[0022] Take Ca(NO 3 ) 2 4H 2 O 0.5807g, Eu(NO 3 ) 3 ·6H 2 O 0.2409g is dissolved in water to form a solution, and Li 2 CO 3 0.0111g is dissolved in nitric acid to form nitric acid solution, and the two solutions are mixed to obtain solution A; get (NH 4 ) 6 Mo 7 o 24 4H 2O 0.5297g was dissolved in water to obtain solution B, and solution A was added while stirring in solution B, then urea 0.9008g and thiourea 0.2283g were added to obtain suspension C; adjust the pH value of suspension C to 4.0 with ammonia water , to obtain a suspension D; move the suspension D into a combustion reaction furnace, and carry out a combustion reaction at 800 ° C. The reaction ends within 4 minutes, and the reaction product is CaMoO according to the present invention. 4 :Eu 3+ , Li + red phosphor.
[0023] figure 1 The real photo of the sample prepared...
Embodiment 2
[0027] According to the chemical formula Ca 1-x MoO 4 :Eu 3+ x , Li + y (x=0.15, y=0.10) Weigh the corresponding medicines.
[0028] Take Ca(NO 3 ) 2 4H 2 O 0.6020g, Eu(NO 3 ) 3 ·6H 2 O 0.2007g, it is dissolved in water, take Li 2 CO 3 0.0111g is dissolved in nitric acid, and two solutions are mixed to obtain solution A; Take (NH 4 ) 6 Mo 7 o 24 4H 2 O 0.5297g was dissolved in water to obtain solution B, and solution A was added while stirring in solution B, then urea 0.7207g and thiourea 0.4567g were added to obtain suspension C; adjust the pH value of suspension C to 3.5 with ammonia water , to obtain a suspension D; move the suspension D into a combustion reaction furnace at 700°C to carry out a combustion reaction, and the reaction ends within 5 minutes to obtain the CaMoO of the present invention. 4 :Eu 3+ , Li + red phosphor.
Embodiment 3
[0030] According to the chemical formula Ca 1-x MoO 4 :Eu 3+ x , Li + y (x=0.20, y=0.15) Weigh the corresponding drugs.
[0031] Take Ca(NO 3 ) 2 4H 2 O 0.5666g, Eu(NO 3 ) 3 ·6H 2 O 0.2676g dissolved in water, take Li 2 CO 3 0.0166g is dissolved in nitric acid, and two solutions are mixed to obtain solution A; Take (NH 4 ) 6 Mo 7 o 24 4H 2 O0.5297g was dissolved in water to obtain solution B, and solution A was added while stirring in solution B, then urea 1.0810g and thiourea 0.1142g were added to obtain suspension C; the pH value of suspension C was adjusted to 4.0 with ammonia water , to obtain a suspension D; move the suspension D into a combustion reaction furnace at 600°C to carry out a combustion reaction, and the reaction ends within 3 minutes to obtain the CaMoO of the present invention. 4 :Eu 3+ , Li + red phosphor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com