Method for determining content and purity of L-carnitine in milk powder
A determination method and carnitine technology are applied in the field of determination of L-carnitine content and purity in milk powder, can solve the problem of inability to distinguish carnitine enantiomers and the like, and achieve the effects of good accuracy, high sensitivity and good separation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, a kind of assay method of L-carnitine content and purity in milk powder is characterized in that comprising the following steps successively:
[0054] 1), sample processing:
[0055] Accurately weigh 16.65g of a commercially available common milk powder sample, dissolve it in 30ml of deionized water, bathe in 50°C water for 15min, and ultrasonic (frequency 30kHz) for 10min to fully dissolve the milk powder, then add deionized water to make up to 100ml.
[0056] Then adjust the pH to 2 with 0.1mol / L hydrochloric acid solution, let it stand for 10 minutes, and then filter it with natural filter paper. The pH of the obtained filtrate was adjusted to 6 with 1mol / L NaOH solution, and after standing for 10 minutes, a second filtration was performed with natural filter paper, and the filtrate obtained from the second filtration was the sample solution.
[0057] 2), prepare standard solution:
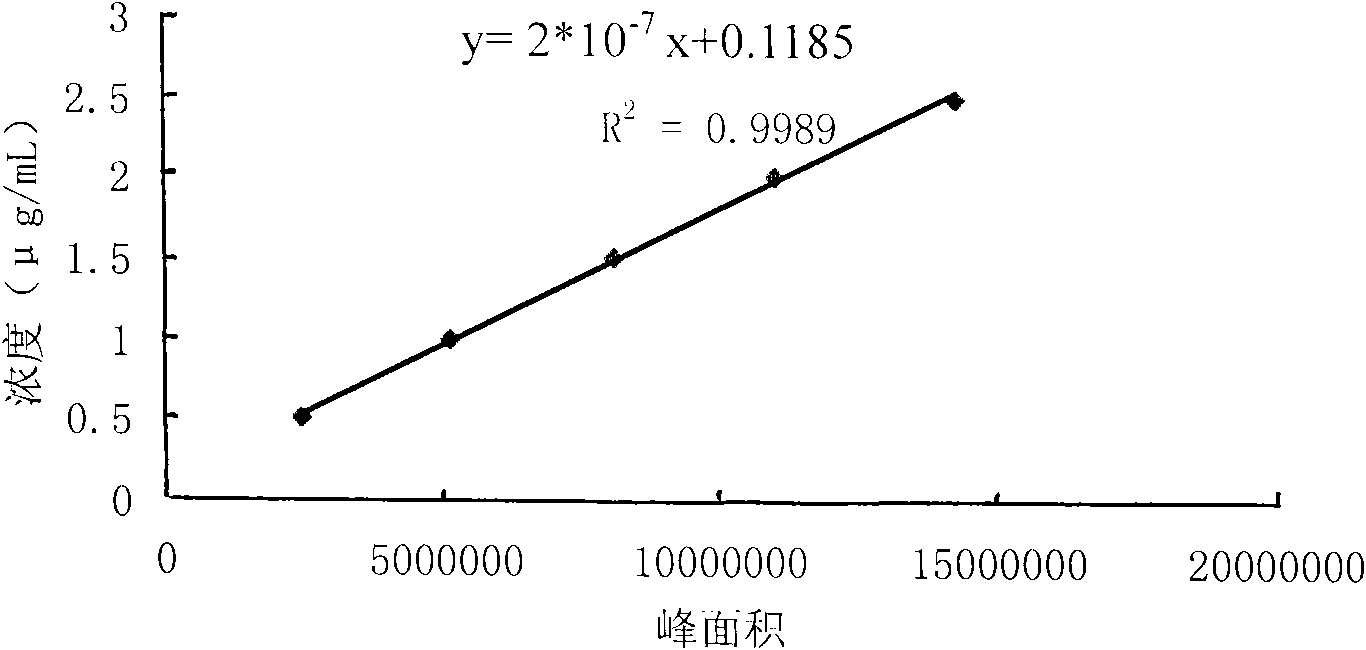
[0058] Weigh 50 mg of L-carnitine standard substance, dissolve it in...
Embodiment 2
[0088] Embodiment 2, a kind of assay method of L-carnitine content and purity in milk powder is characterized in that comprising the following steps successively:
[0089] 1), sample processing:
[0090] Accurately weigh 14.95g of commercially available infant formula milk powder No. 1 sample, dissolve it in 30m deionized water, bathe in 50°C water for 15min, ultrasonic (frequency 30kHz) 10min, the milk powder is fully dissolved, add deionized water to make up to 100ml.
[0091] Then adjust the pH to 1.9 with 0.1mol / L hydrochloric acid solution, let it stand for 10min, and then filter it with natural filter paper. Adjust the pH of the obtained filtrate to 6.1 with 1 mol / L NaOH solution, and perform a second filtration with natural filter paper, and the filtrate obtained from the second filtration is the sample solution.
[0092] Step 2) to step 6) are all the same as in Example 1.
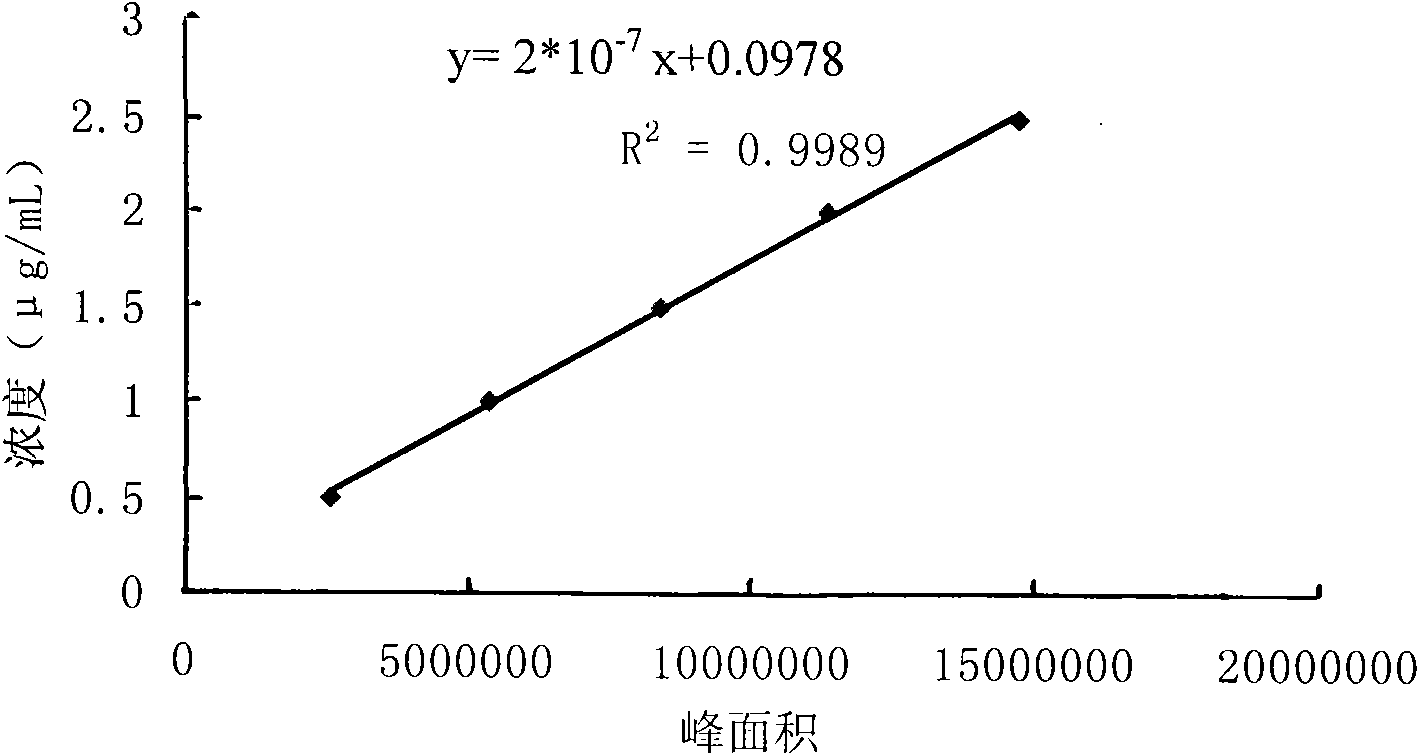
[0093] Thereby obtain respectively the concentration cL=(812098*2*10 of L-carnitine in the sa...
Embodiment 3
[0097] Example 3. The commercially available infant formula milk powder No. I sample in Example 2 was changed to the commercially available infant formula milk powder No. II sample, and 15.63 g of the sample was accurately weighed.
[0098] Step 1) to step 6) are all the same as in Example 2.
[0099] Thereby get respectively the concentration cL=(848302*2*10 of L-carnitine in the sample solution -7 +0.0978)*10*1000 / 60=44.57 μg / ml, the concentration of D-carnitine cD=0 μg / ml;
[0100] 7), obtain the content and purity of L-carnitine in the sample:
[0101] The calculation formula is the same as in Example 1.
[0102] The results were as follows: the content of L-carnitine in No. II sample of infant formula milk powder was 28.52mg / 100g, and D-carnitine was not detected.
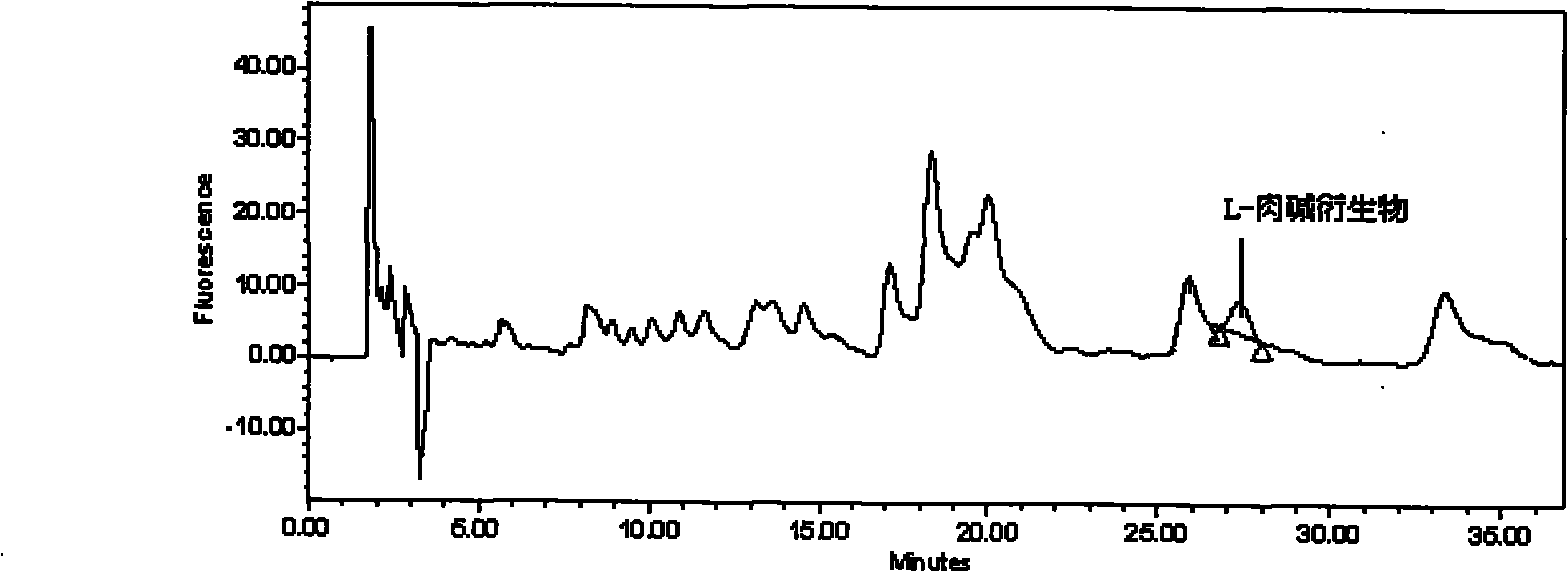
[0103] In order to prove the correctness of the conclusion of the present invention, utilize the existing high-performance liquid chromatography ultraviolet analysis method to completely the common milk pow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com