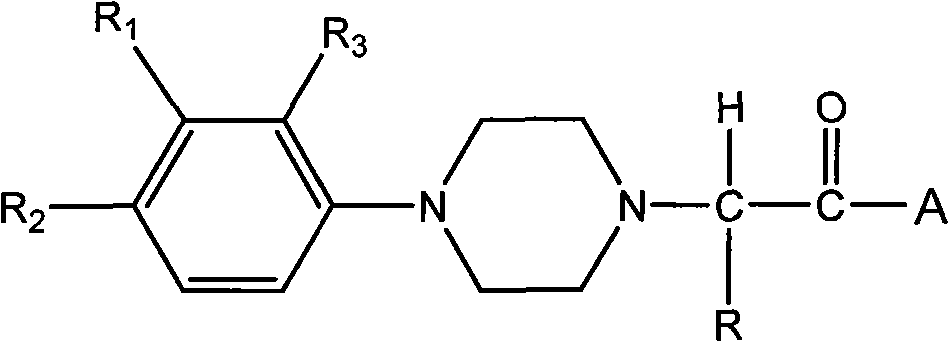
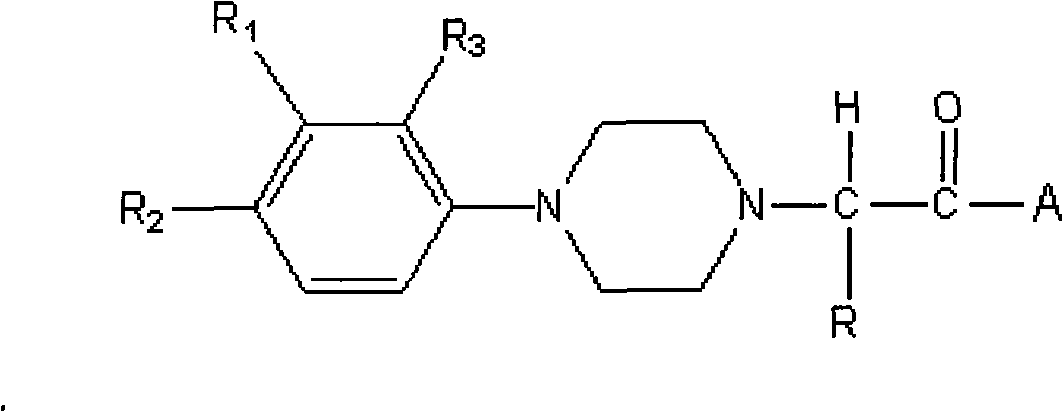
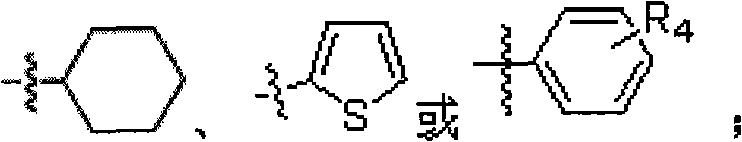Substituted phenyl piperazinyl aralkylone derivatives and application thereof to preparation of analgesics
A technology of phenylpiperazine and aralkylone, which is applied in the field of substituted phenylpiperazine aralkylone derivatives, can solve problems such as the reduction of addictive gastric motility, and achieve the effect of strong anti-pain writhing response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0087] 1) Preparation of α-bromophenone
[0088] 0.1 mol of aryl alkanone was dissolved in 200 ml of a mixed solvent of chloroform and ethyl acetate (volume ratio 1:1), and 0.2 mol of solid copper bromide was added under stirring at room temperature, and the reaction was refluxed for 12 hours. Cool the reaction solution to room temperature, filter, and concentrate the filtrate to dryness. The remaining oil is extracted with petroleum ether (2x100ml) to remove insoluble matter. The petroleum ether phases are combined and evaporated to dryness to obtain an oil. Yield: 40-94%.
[0089] 2) Preparation of 1-substituted phenyl-4-aroylalkylpiperazine hydrochloride
[0090] Substituted phenylpiperazine (0.01mol) and a-bromoaryl alkanone (0.012mol) were dissolved in 50ml of acetone solvent, anhydrous potassium carbonate (4.15g, 0.03mol) was added, and the temperature was raised to reflux for 5 hours. Cool the reaction solution, filter, evaporate the filtrate to dryness, add 150ml of e...
Embodiment 1
[0092] Preparation of 1-(4-methoxyphenyl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone hydrochloride (I-1)
[0093] Using p-methoxyacetophenone as raw material, prepare a-bromo-p-methoxyacetophenone according to the synthesis and post-treatment methods in the general method. Take a-bromo-p-methoxyacetophenone (0.66g, 2.86mmol) and 3-trifluoromethylphenylpiperazine (0.60g, 2.6mmol), dissolve them in 50ml of acetone solvent, add anhydrous potassium carbonate (1.08g, 7.8mmol), heated and refluxed for 5 hours. According to the post-treatment operation in the general method, 1-(4-methoxyphenyl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone hydrochloride was obtained Salt 0.86g, yield 79.26%. Melting point: 216.7-218.7℃, MS(m / s): 379.0[M+1] + .
[0094] 1 HNMR (DMSO-d6): δ3.33-3.96 (m, 8H, piperazine-H), 3.87 (s, 3H, -OCH 3 ), 5.12 (s, 2H, -CH2-), 7.12-7.98 (m, 8H, Ar-H).
Embodiment 2
[0096] Preparation of 1-(3-methoxyphenyl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone hydrochloride (I-2)
[0097] Using 3-methoxyacetophenone as a raw material, prepare a-bromo-3-methoxyacetophenone according to the synthesis and post-treatment methods in the general method. Take a-bromo-3-methoxyacetophenone (0.66g, 2.86mmol) and 3-trifluoromethylphenylpiperazine (0.60g, 2.6mmol), dissolve in 50ml of acetone solvent, add anhydrous Potassium carbonate (1.08g, 7.8mmol) was heated and refluxed for 5 hours. According to the post-treatment operation in the general method, 1-(3-methoxyphenyl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone hydrochloride was obtained Salt 0.82g, yield 75.93%. Melting point: 219.9-222.3℃, MS(m / s): 379.1[M+1] + .
[0098] 1 HNMR (DMSO-d6): δ3.36-3.96 (m, 8H, piperazine-H), 3.84 (s, 3H, -OCH 3 ), 5.22(s, 2H, -CH 2 -), 7.14-7.59 (m, 8H, Ar-H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com