Preparation and applications of paeonol controlled-release preparation
A sustained-release preparation, the technology of paeonol, which is applied in the direction of anti-inflammatory agents, non-central analgesics, medical preparations of non-active ingredients, etc., can solve the problems of short elimination half-life, high frequency of medication, and difficult storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
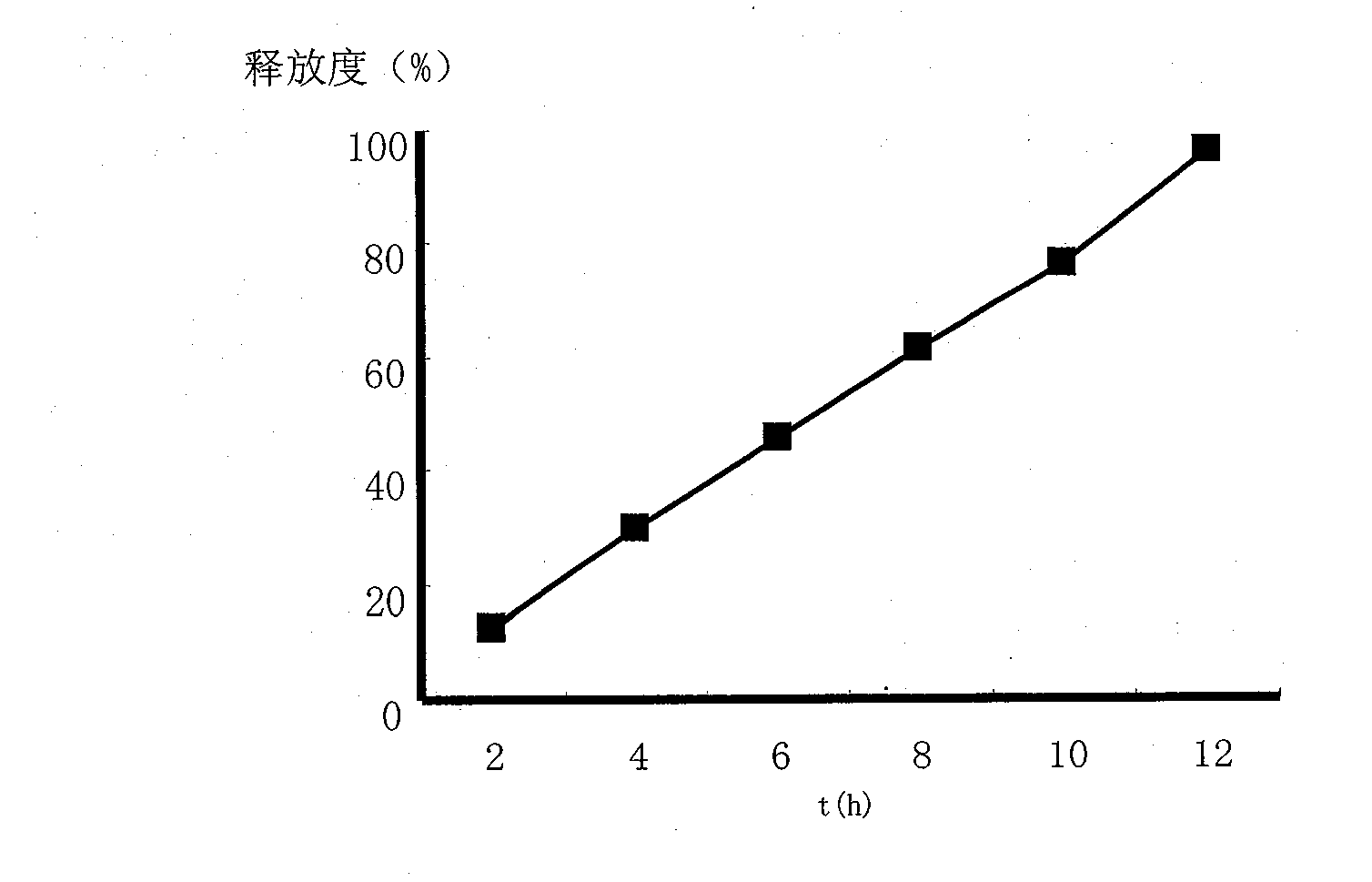
[0047] Embodiment 1: Paeonol sustained-release tablet
[0048] [Prescription] Paeonol 60mg
[0049] HPMC K4M 40mg
[0050] HPMC K100M 20mg
[0051] Lactose 84mg
[0053] [Preparation process] pass paeonol through 100 mesh sieve and HPMC K4M 、HPMC K100M , lactose and other auxiliary materials are mixed evenly, and an appropriate amount of magnesium stearate is added as a lubricant. After mixing, the whole powder is directly compressed into tablets, and the product is obtained.
[0054] [Dissolution] The method refers to "Chinese Pharmacopoeia" 2005 Edition Part II Dissolution Determination Method (Appendix XC Method 3) and Dissolution Determination Method (Appendix XD Method 1), using 900ml distilled water as the release medium, and the rotation speed is 100r min -1 , temperature 37.0°C. At 2, 4, 6, 8, 10, and 12 hours, 6ml samples were taken respectively and distilled aqueous solution of the...
Embodiment 2
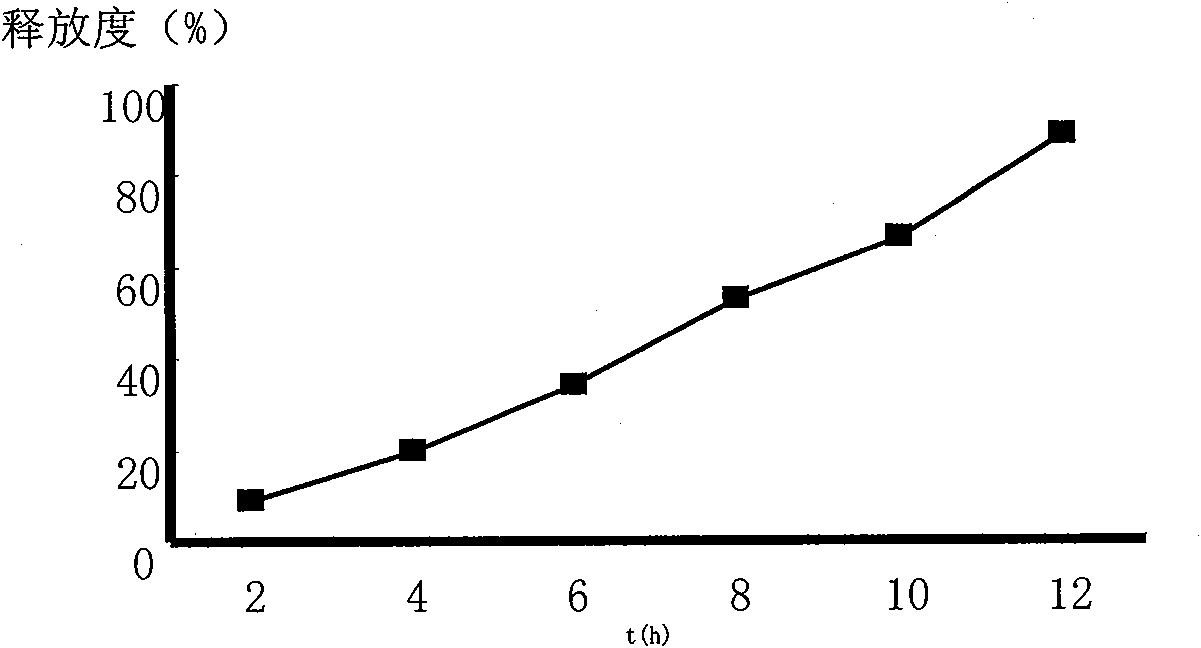
[0055] Embodiment 2 paeonol sustained release tablet
[0056] [Prescription] Paeonol 60mg
[0057] HPMC K4M 40mg
[0058] HPMC K100M 20mg
[0059] Lactose 108mg
[0061] [Preparation process] pass paeonol through 100 mesh sieve and HPMC K4M 、HPMC K100M , lactose and other auxiliary materials are mixed evenly, and an appropriate amount of magnesium stearate is added as a lubricant. After mixing, the whole powder is directly compressed into tablets, and the product is obtained.
Embodiment 3
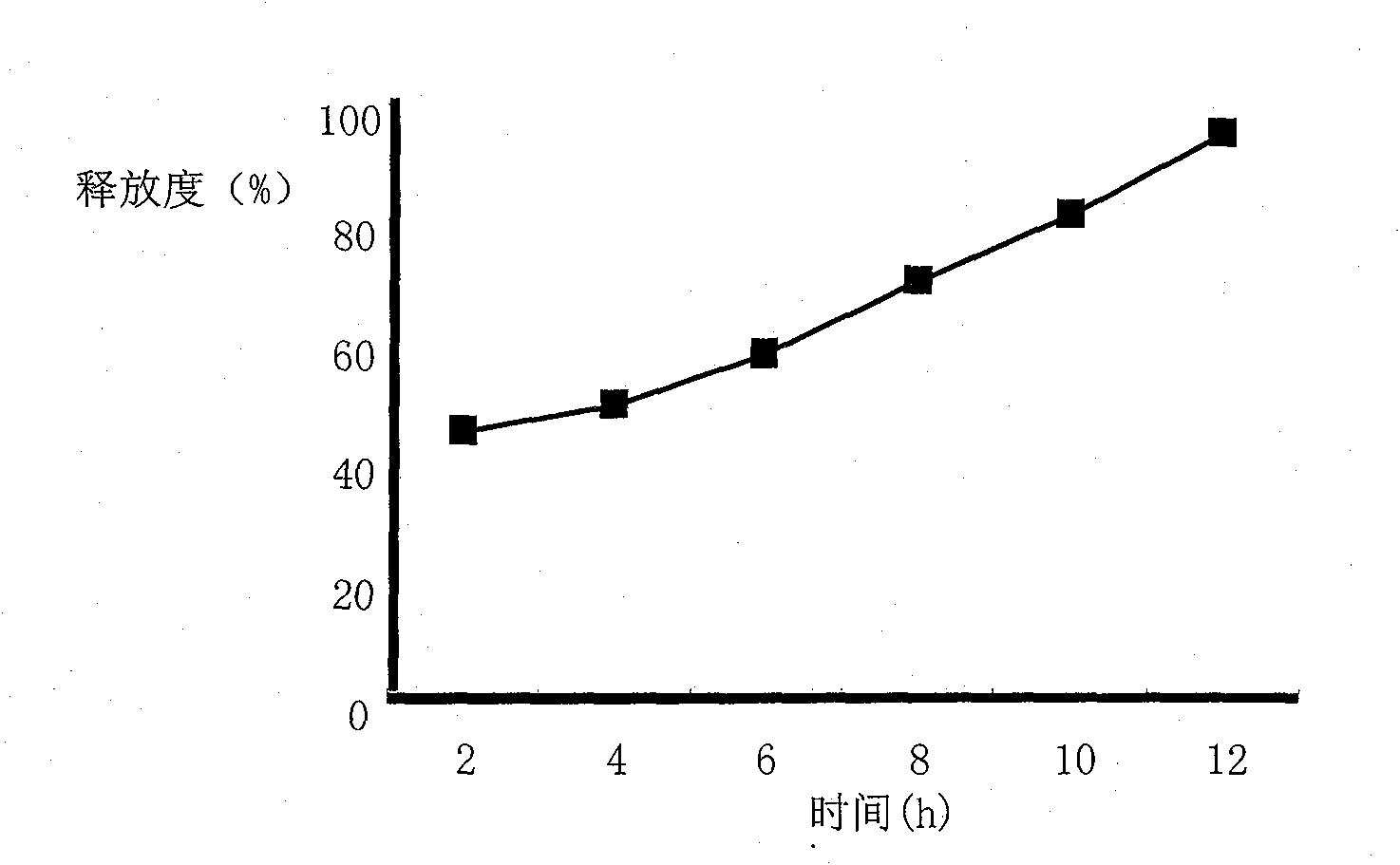
[0062] Embodiment 3: Paeonol sustained release tablet
[0063] [Prescription] Paeonol 60mg
[0064] HPMC K4M 30mg
[0065] HPMC K100M 30mg
[0066] Lactose 84mg
[0068] [Preparation process] Paeonol is passed through a 100 mesh sieve and mixed with HPMC K4M 、HPMC K100M , lactose and other auxiliary materials are mixed evenly, and an appropriate amount of magnesium stearate is added as a lubricant. After mixing, the whole powder is directly compressed into tablets, and the product is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com