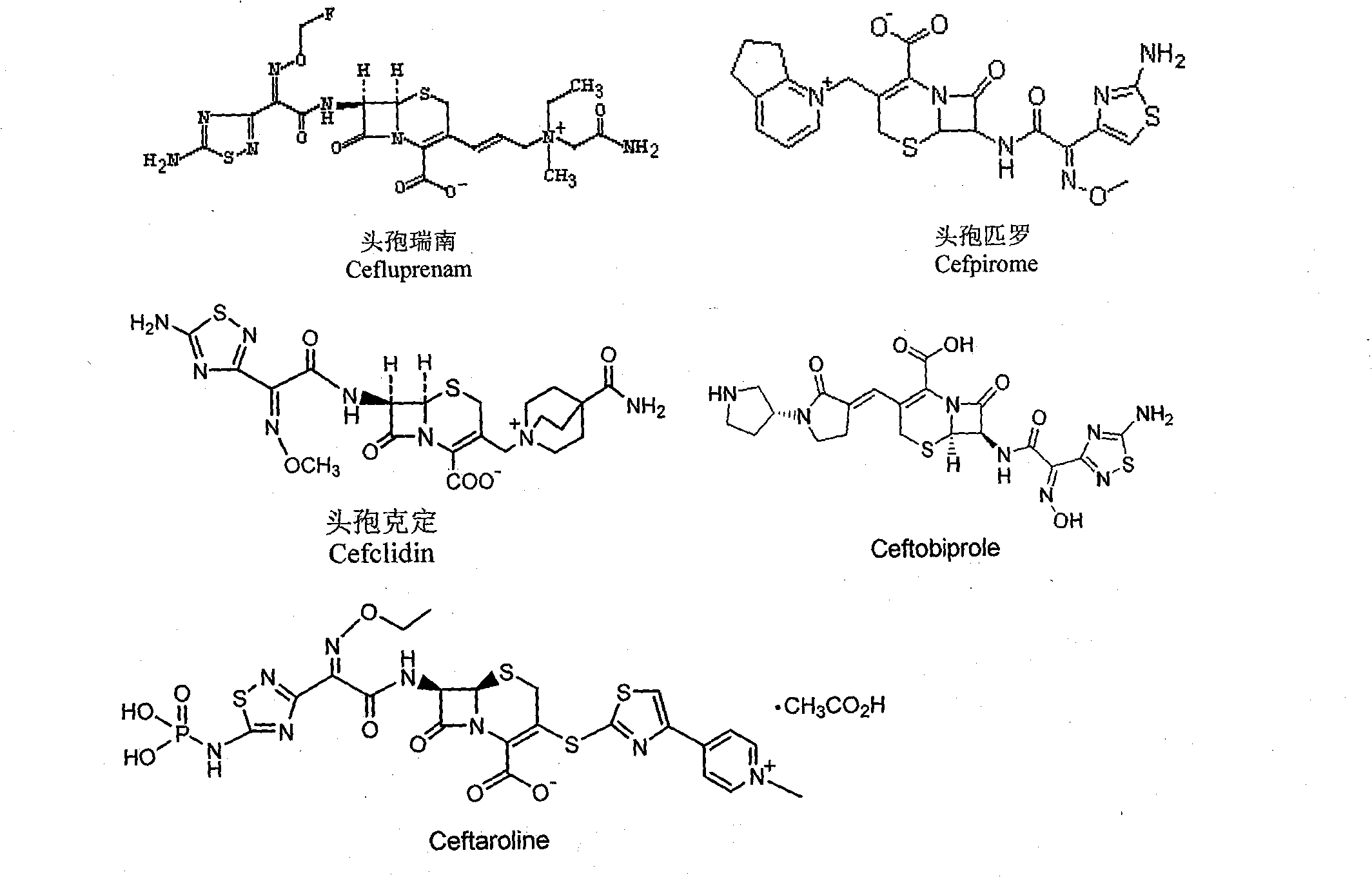
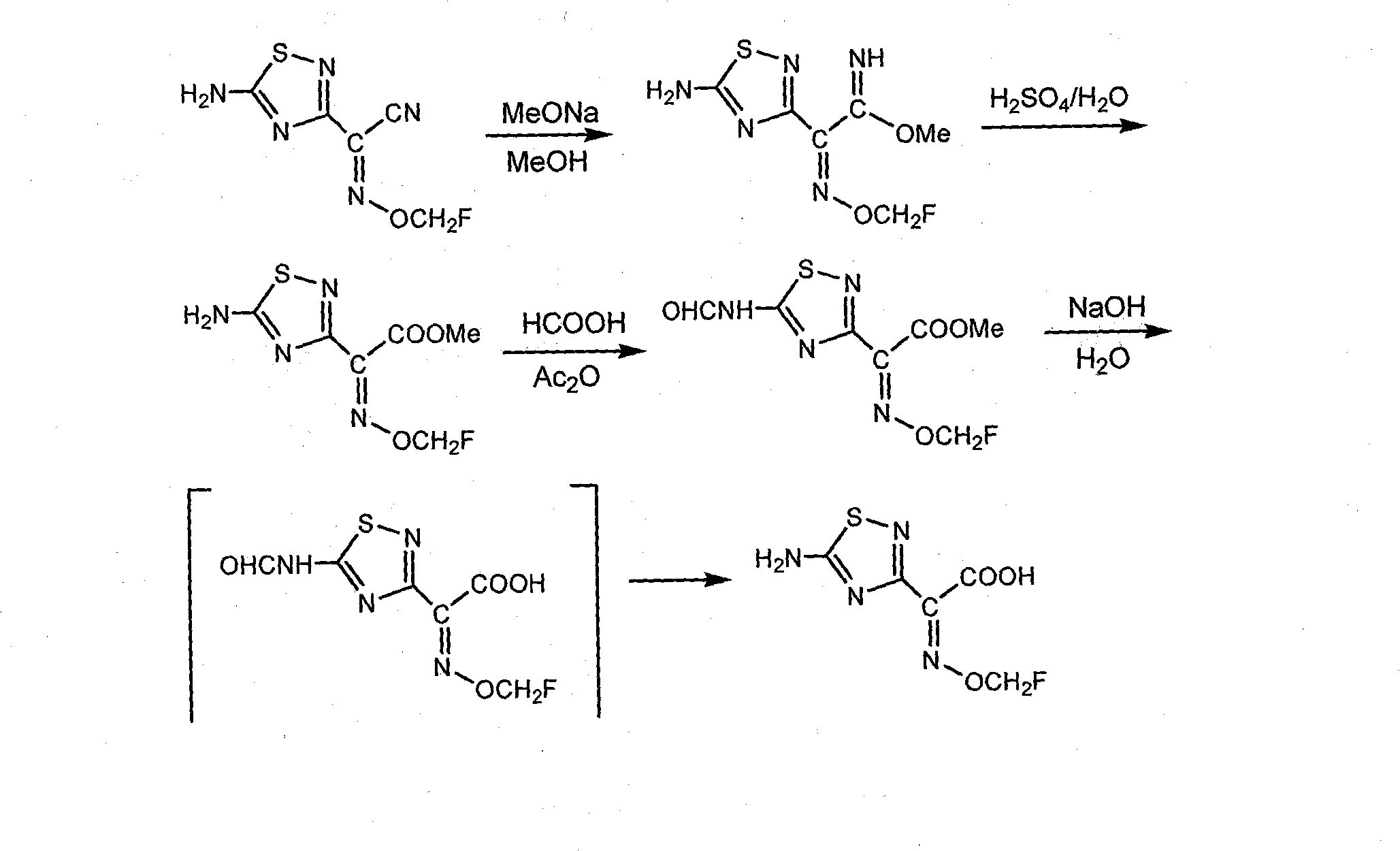
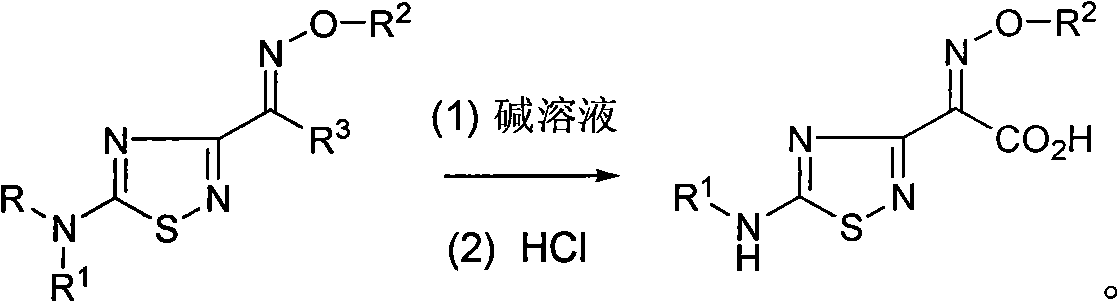
Preparation method of 1,2,4-thiadiazole oximido acetic acid compound
A technology for thiadiazoloxime and compounds, which is applied in the field of preparation of 1,2,4-thiadiazoloxime acetic acid series compounds, which can solve complex operations and shorten the operation steps and process steps of thiadiazoloxime acetic acid series compounds To achieve the effect of high reaction yield, unique method and principle, and shortened operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055] Synthesis of 2-methoxyimino-2-(5-amino-1,2,4-thiadiazole-3)-acetic acid: 2-methoxyimino-2-(5-formylamino-1 , 2,4-thiadiazole-3)-methyl acetate (265g, 97%, 1.0mol) was added to a 5L there-necked flask, and then 3L (3.0mol) of 1.0M LiOH aqueous solution was added to the reaction flask, and the temperature was raised to React at 50-60°C for 12 hours. TLC tracking analysis, after the reaction is completed, lower to room temperature, adjust the pH to 5 with concentrated hydrochloric acid, add 50g of activated carbon to decolorize, then adjust the pH to 1 with concentrated hydrochloric acid, a large number of white crystals precipitate, filter and wash with a small amount of cold methanol, and dry to obtain white Solid 153g, yield 80%, content (HPLC) 97%, H 1 NMR (500Hz, DMSO-d e ), δ3.93 (3H, s), 8.21 (2H, s), mp.175~178°C (dec).
Embodiment 2
[0057]
[0058] Synthesis of 2-ethoxyimino-2-(5-amino-1,2,4-thiadiazole-3)-acetic acid: 2-ethoxyimino-2-(5-formylamino-1 , 2,4-thiadiazole-3)-acetonitrile (520g, 95%, 2.1mol), 3.5L of water was added in a 5L four-necked flask, and 383g (4.60mol) of 48% aqueous sodium hydroxide solution was added under stirring, and the temperature was raised to Reaction at 50-60°C. TLC tracking analysis, after the reaction (about 12 hours), cool down, adjust the pH to 4 with concentrated hydrochloric acid, add 50 g of activated carbon for decolorization, then adjust the pH to 1 with concentrated hydrochloric acid, extract with ethyl acetate (2LX2), and evaporate the solvent to dryness under reduced pressure After adding 1.5L acetonitrile for recrystallization, 178g of light yellow solid powder product was obtained, yield 39%, content (HPLC) 92%, H 1 NMR (500Hz, DMSO-d e ), δ1.22 (3H, t, J=7Hz,), 4.17 (2H, q, J=7Hz), 8.17 (NH2, 2H, bs), mp.160~162°C (dec).
Embodiment 3
[0060]
[0061] Synthesis of 2-ethoxyimino-2-(5-amino-1,2,4-thiadiazole-3)-acetic acid: 2-ethoxyimino-2-(5-formylamino- 1,2,4-thiadiazole-3)-acetonitrile (258g, 95%, 1mol) was added to a 5L three-necked flask, then 3.5L LiOH (1.2M) aqueous solution was added to the reaction flask, and hexadecyl Trimethylammonium chloride (7.8g), 120g 30% H 2 o 2 Add 120g 30% H 2 o 2 , 30°C-35°C stirring reaction for 6 hours. The temperature was raised to 70°C-75°C for 12 hours, followed by TLC. After cooling down to 5°C, the reaction liquid was acidified with concentrated hydrochloric acid to pH 1.5, and a large amount of white crystals were precipitated. The solid was filtered and washed with a small amount of cold methanol, and the product was vacuum-dried to obtain 171 g of a white solid product with a content (HPLC) of 97%. Yield 81%, H 1 NMR (500Hz, DMSO-d e ), δ1.22(3H, t, J=7Hz,), 4.17(2H, q, J=7Hz), 8.17(NH 2 , 2H, bs), mp.163~165℃(dec)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com