Synthetic peptide vaccine for swine fever and application thereof
A technique for synthesizing peptide vaccines and swine fever, applied to non-active ingredient medical preparations, active ingredient-containing medical preparations, peptides, etc., can solve problems such as inability to effectively protect pigs, achieve easy control of production quality, and improve immunity The effect of originality and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The branched peptides and linear peptides shown below were artificially synthesized by conventional methods in the art, and named E28 and E26, respectively. The purity of the synthetic peptides is above 90%. Dilute the synthetic peptides with sterile PBS to a peptide solution with a concentration of 1 microgram per microliter, and then mix the peptide solution with an equal amount of protein adjuvant (such as Freund's adjuvant, etc.) Mixed, emulsified into a vaccine, used as an immunogen for immunizing animals.
[0059] Branched Peptide-E28
[0060] Linear peptide - E26ISISEIKGVIVHKIEGILFKKCTAVSPTTLRTEVVK
[0061] Select 15 healthy rabbits weighing about 2 kg, and divide them into 3 groups, 5 rabbits in each group. Rabbits No. 8, 9, and 10) were immunized with branched peptide E28, and the third group (rabbits No. 11, 12, 13, 14, and 15) were immunized with PBS as a negative control. The peptide vaccine immunization dose is 50 micrograms each time, with a total vol...
Embodiment 2
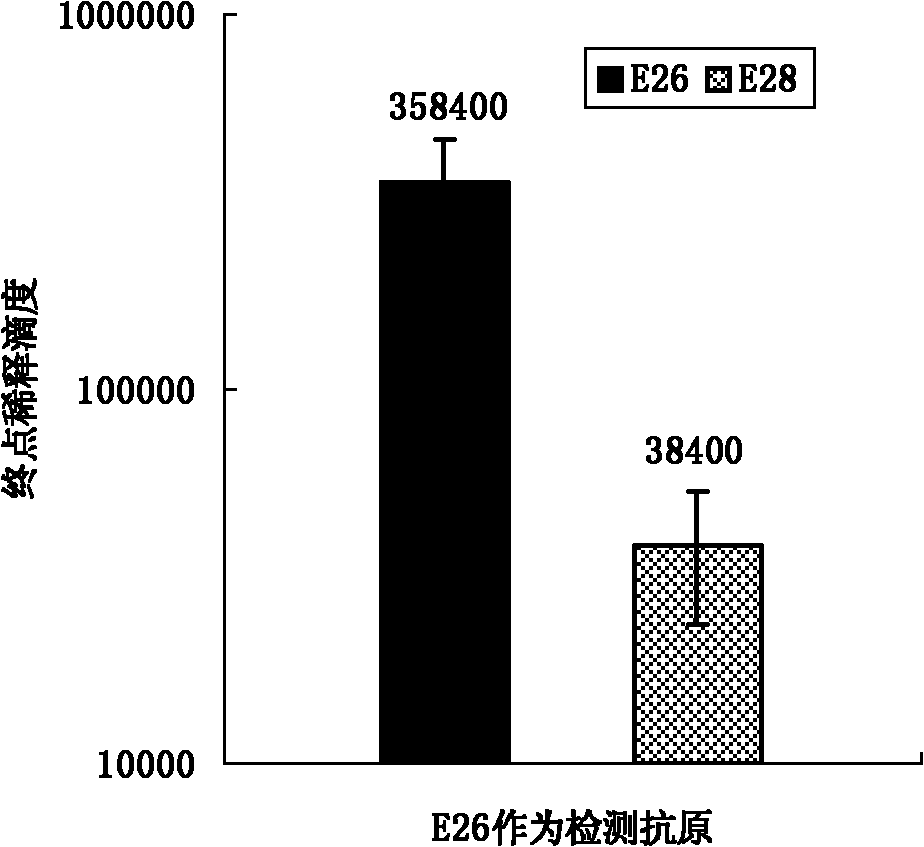
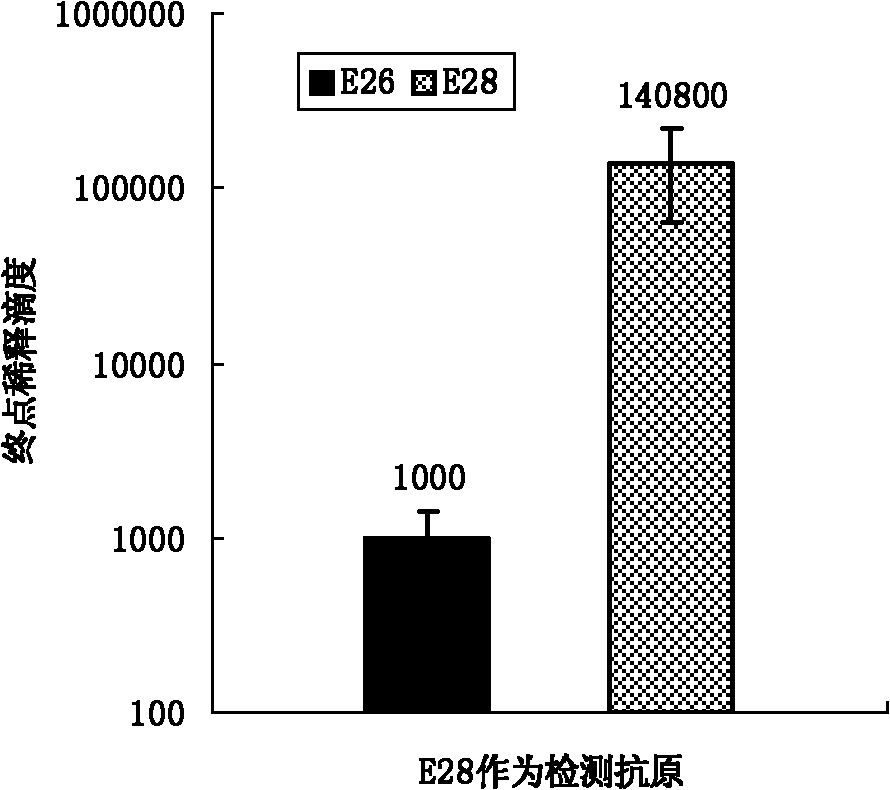
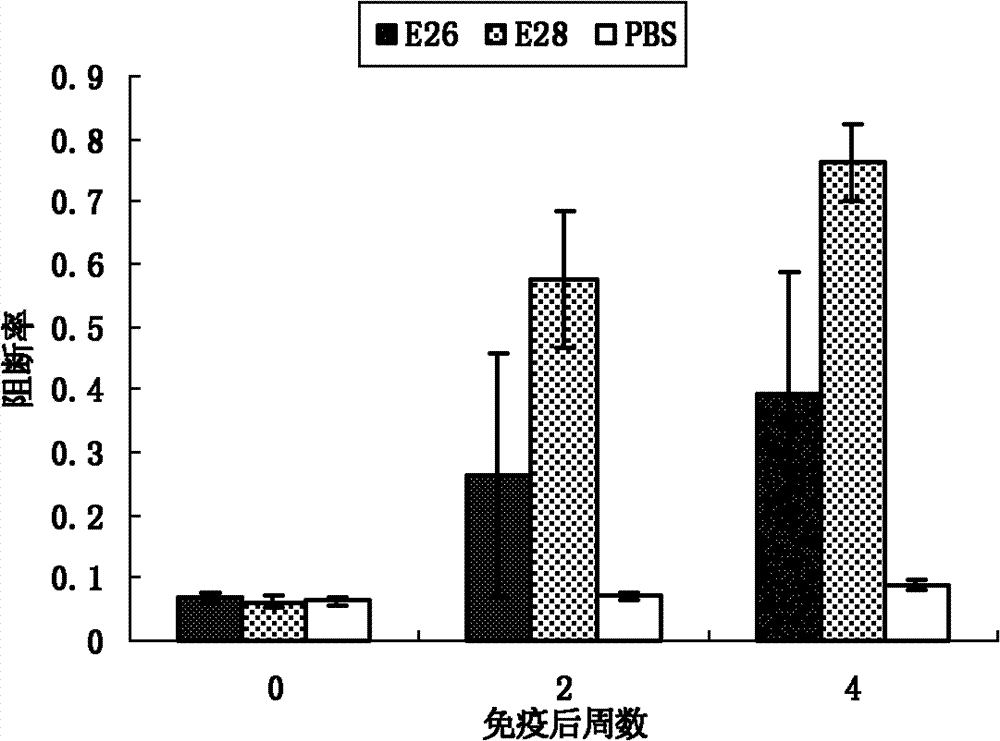
[0067] Peptide vaccines were prepared as in Example 1 and used as immunogens to immunize animals. Select 20 healthy piglets negative for swine fever antibody about 35 days old and divide them into 4 groups, 5 pigs in each group. The first group is immune to the linear peptide E26, the second group is immune to the branched peptide E28 prepared by the present invention, and the remaining 2 groups are respectively Immune attenuated classical swine fever vaccine (HCLV) and PBS, as positive control and negative control. The dose of peptide vaccine immunization is 300 micrograms each time, the total volume is 1 ml, multi-point intramuscular injection, immunization 2 times, with an interval of 2 weeks. The first emulsification with Freund's complete adjuvant and the second emulsification with Freund's incomplete adjuvant. Before immunization and after immunization, blood was collected every week to separate serum, and the serum antibody level was detected by peptide ELISA and IDEXX...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com