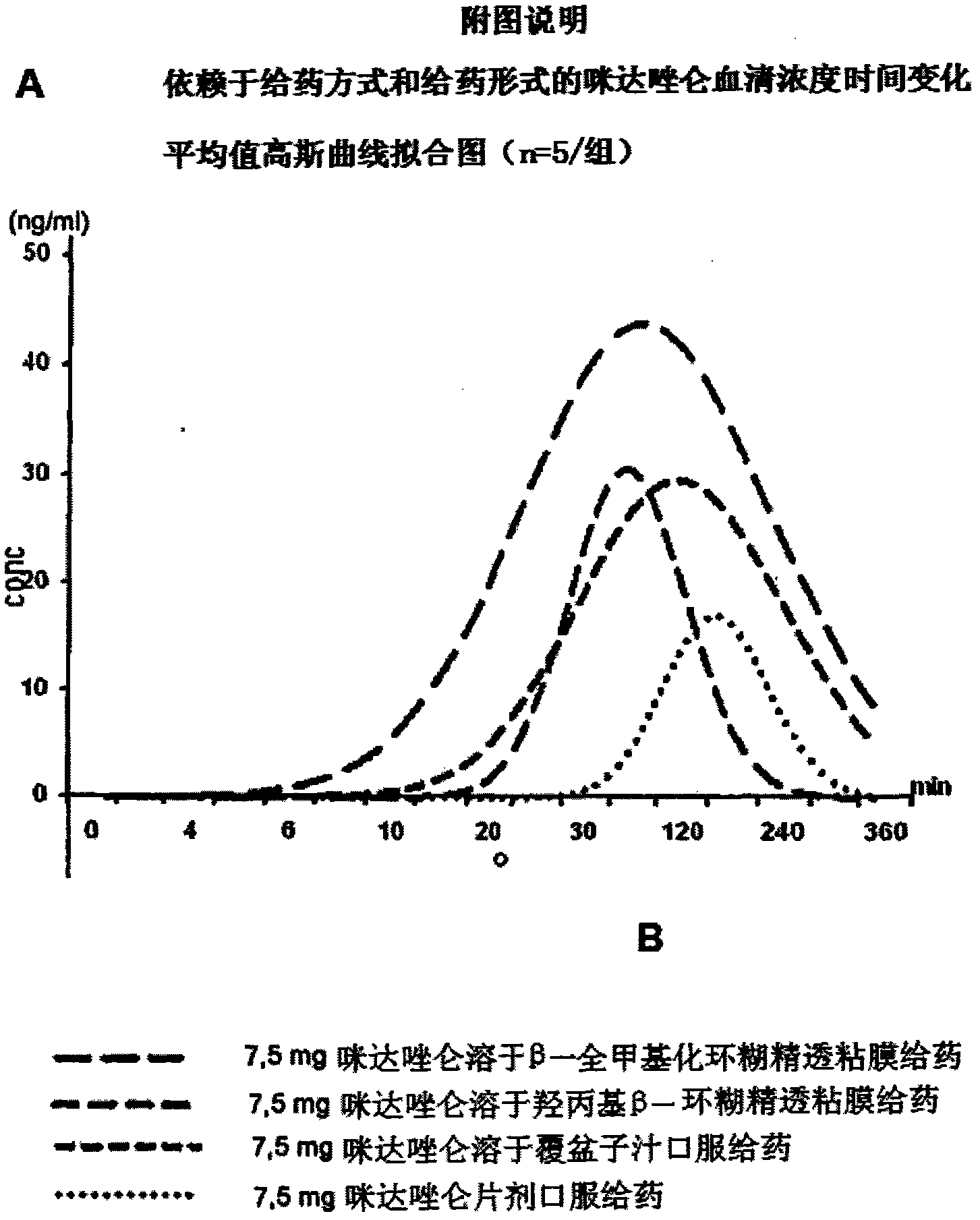
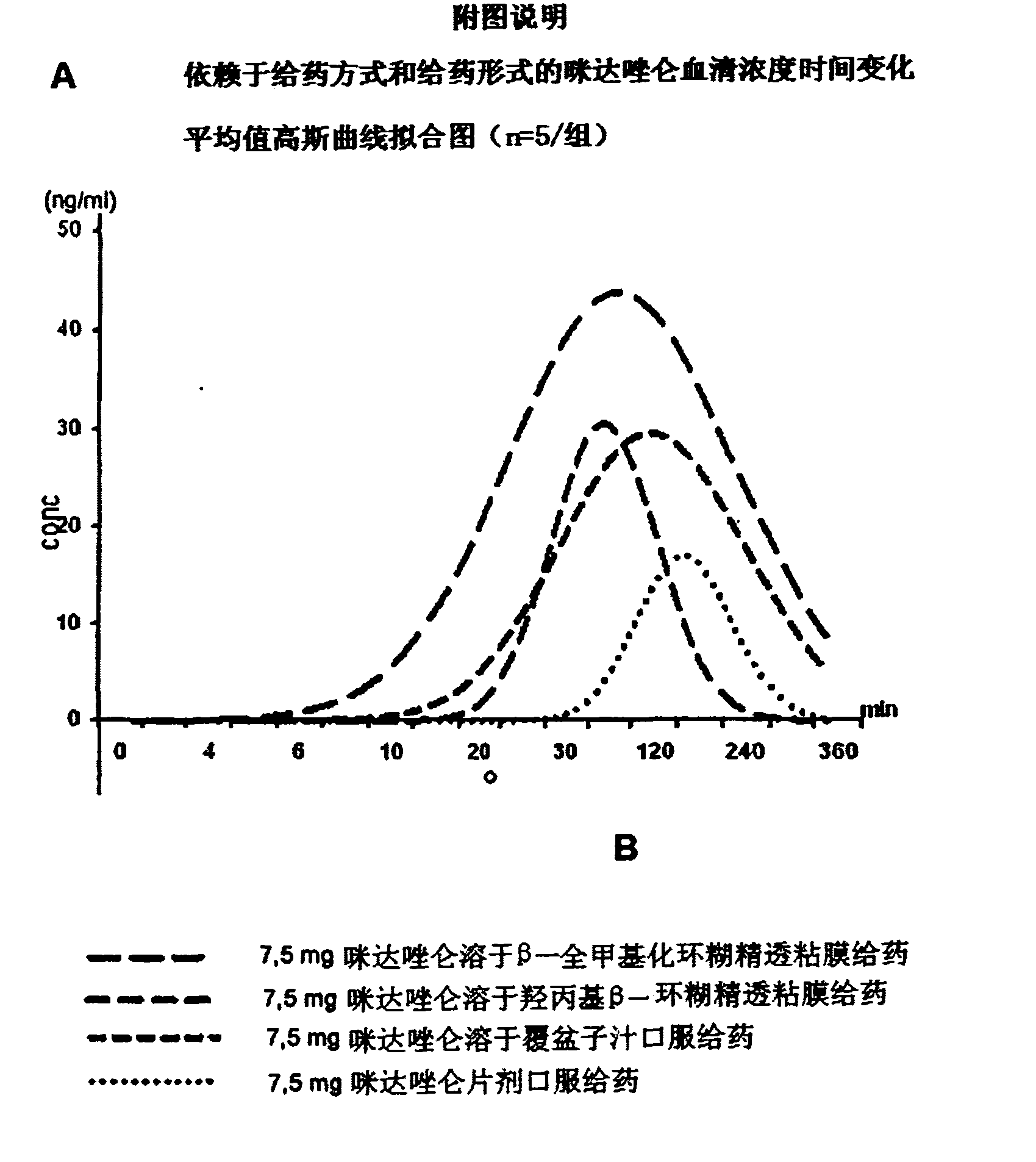
Pharmaceutical preparation comprising permethylated cyclodextrin
A composition, cyclodextrin technology, applied in the directions of drug combination, active ingredients of hydroxyl compounds, drug delivery, etc., can solve the problems of incorrect dose, uncoordinated, difficult to calculate, etc. Improve, fast and reliable transmission, improve the effect of transmission speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Midazolam compositions for transmucosal administration.
[0044] A 1% midazolam composition for transmucosal administration is formulated as follows
[0045] Midazolam 10mg
[0046] Fully methylated β-cyclodextrin (methylation degree 3) 150mg
[0047] Hypromellose 400 (hypromellose) 1mg
[0048] Concentrated phosphoric acid 2.6mg
[0049] NaOH 10%: Appropriate amount, adjust the pH value to 4.2
[0050] Potassium sorbate 1.4mg
[0051] water to 1ml
[0052] Hypromellose 400 is a hydroxypropylmethylcellulose with a molecular weight of approximately 400 that acts as a mucosal wetting agent. The pH of the prepared solution was adjusted to 4.2. The mass ratio of cyclodextrin and pharmaceutical active substance in this embodiment is 15:1, the mass ratio of cyclodextrin of the present invention and pharmaceutical active substance is preferably 50:1 to 10:1, more preferably 40:1 to 12:1 , more preferably 20:1 to 12:1.
[0053] A group of five subjects were each given ...
Embodiment 2
[0063] Intravenous propofol composition
[0064] Two 1% formulations of propofol were used. A kind of is the preparation of prior art, the fat emulsion of business name Diprivan, and emulsion matrix is made up of soybean oil, glycerol, lecithin, contains 1% propofol in the emulsion matrix.
[0065] Formula of the present invention:
[0066] Propofol 10mg
[0067] Permethylated β-cyclodextrin 80.2mg
[0068] Concentrated phosphoric acid 2.mg
[0069] NaOH 10%: Appropriate amount, adjust the pH value to 6.8
[0070] water to 1ml
[0071] Hereinafter, this formula is referred to as CD-Prppofol (CD propofol).
[0072] Each of twelve Göttingen minipigs weighing between 48 and 53 kg were randomly anesthetized with Diprivan and CD-Prppofol (CD Propofol). Injection is performed through a peripheral venous catheter.
[0073] The depth of anesthesia is monitored by the depth of anesthesia monitor CSM (cerebral state monitoring) and the corresponding depth of anesthesia index C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com