Taxol complex preparation drug
A compound preparation, paclitaxel technology, applied in the field of medicine, can solve the problems of uncontrolled slow release time of the inner layer drug, injection toxicity, inner layer damage, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
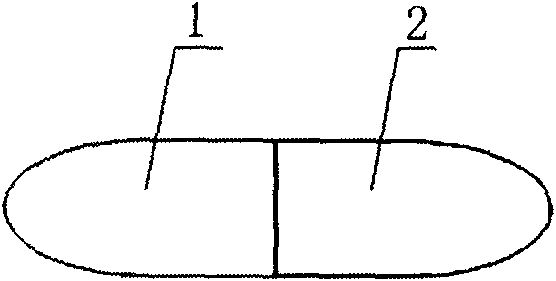
[0033] like figure 1 As shown in the figure, the paclitaxel compound preparation drug, part of which is paclitaxel anticancer drug sustained-release capsule 1, and the other part is cyclosporine A rapid-release capsule 2; the two parts of the capsule are pressed together to become one and directly applied.
[0034] The preparation method is as follows:
[0035] 1) Preparation of paclitaxel anticancer drug sustained-release capsules: 1.5 grams of paclitaxel was dissolved in 10 grams of D-alpha-polyethylene glycol 1000 succinate (TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate), followed by adding 5 grams of caprylic acid A mixture of polyethylene glycol glycerides and capric polyethylene glycol glycerides, 3 grams of sorbitan monooleate (Sorbitan monooleate), 0.2 grams of Ascorbyl palmitate (Ascorbyl palmitate) and 1 gram of absolute ethanol. This formula is mixed well for 100 unit doses. The obtained mixture and sustained-release capsules are pelleted through an ...
Embodiment 2
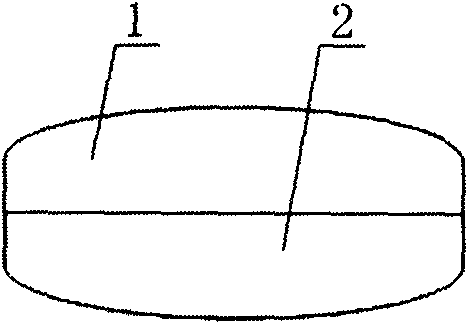
[0040] like image 3 As shown, a paclitaxel compound preparation drug, part of which is paclitaxel anticancer drug sustained-release capsule 1, and the other part is cyclosporine A rapid-release capsule 2; the two parts of the capsule are pressed together, and then coated with a layer of Capsule 3, becomes a composite capsule;
[0041] The preparation method is as follows:
[0042] 1) Preparation of paclitaxel anticancer drug capsules: 1 g of docetaxel was dissolved in 17 g of D-alpha-polyethylene glycol 1000 succinate (TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate), followed by adding 3.5 g of caprylic acid A mixture of polyethylene glycol glycerides and capric polyethylene glycol glycerides, 2 grams of sorbitan monooleate (Sorbitan monooleate), 0.1 grams of Ascorbyl palmitate (Ascorbyl palmitate) and 0.5 grams of absolute ethanol. This formula is mixed well for 100 unit doses. The obtained mixture and capsules are pelleted through an automatic soft capsule m...
Embodiment 3
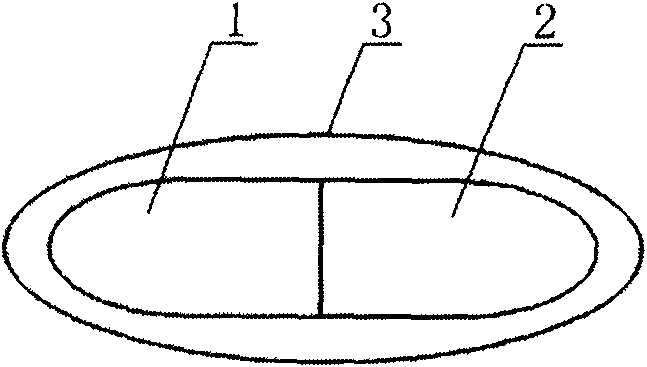
[0047] like Figure 5 As shown in the figure, a paclitaxel compound preparation drug, one part is the paclitaxel anticancer drug capsule 1, and the other part is the cyclosporine A capsule 2; the two parts of the capsule are pressed together to become one, and can be directly applied.
[0048] Preparation:
[0049] 1) Preparation of paclitaxel anticancer drug capsules: 1 g of docetaxel was dissolved in 17 g of D-alpha-polyethylene glycol 1000 succinate (TPGS, D-alpha-tocopheryl polyethylene glycol 1000 succinate), followed by adding 3.5 g of caprylic acid A mixture of polyethylene glycol glycerides and capric polyethylene glycol glycerides, 2 grams of sorbitan monooleate (Sorbitan monooleate), 0.1 grams of Ascorbyl palmitate (Ascorbyl palmitate) and 0.5 grams of absolute ethanol. This formula is mixed well for 100 unit doses. The obtained mixture and capsules are pelleted through an automatic soft capsule machine, rolled and shaped, dried at 20-30° C., selected for pills, wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com