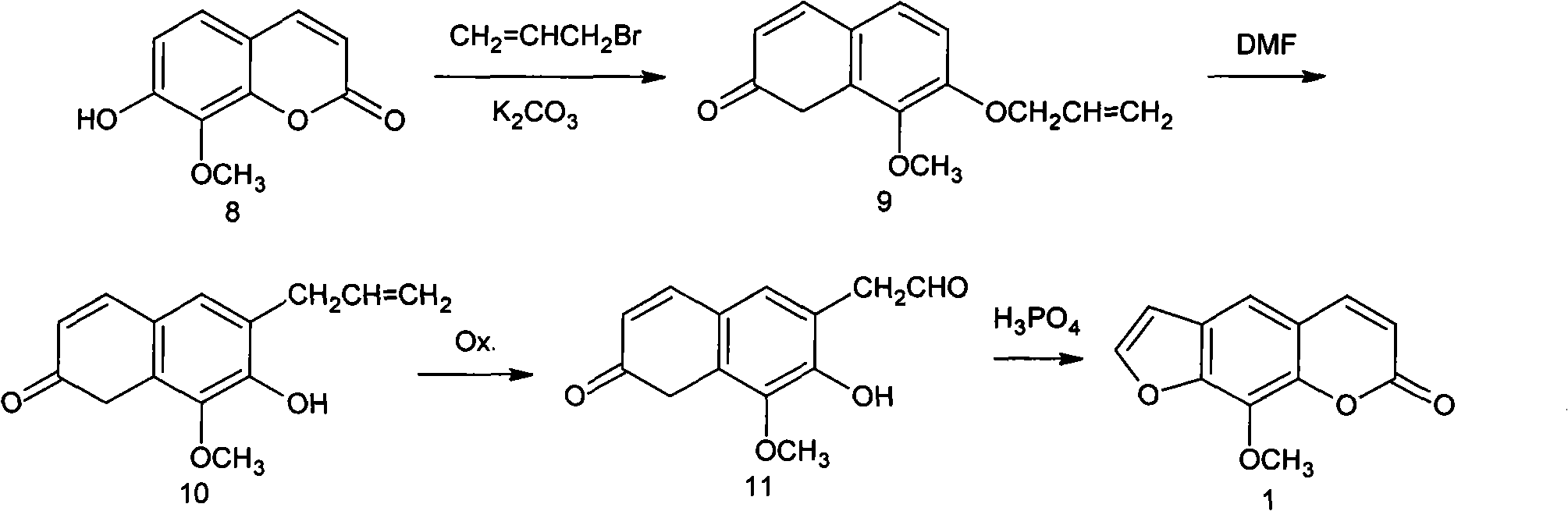
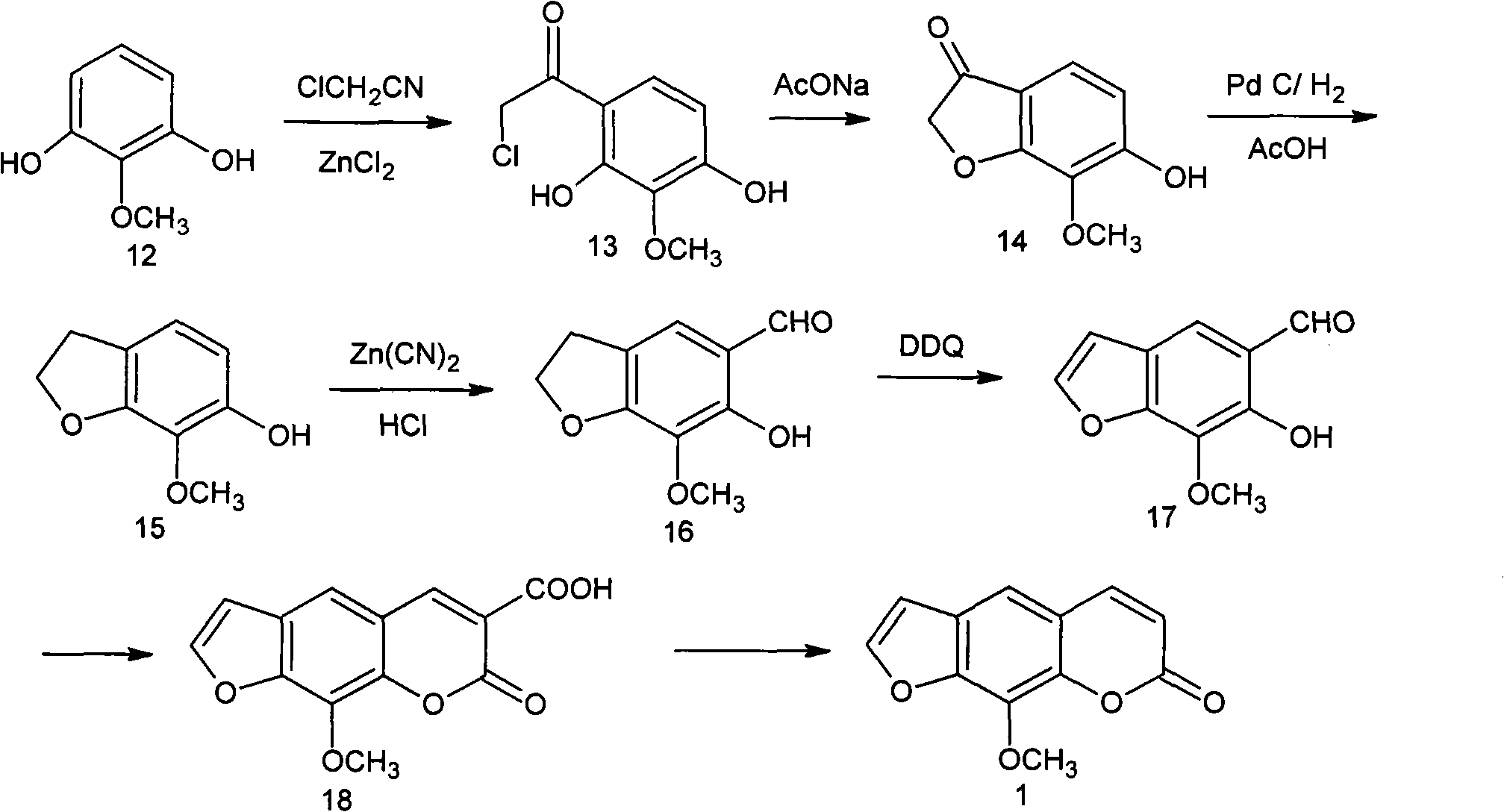
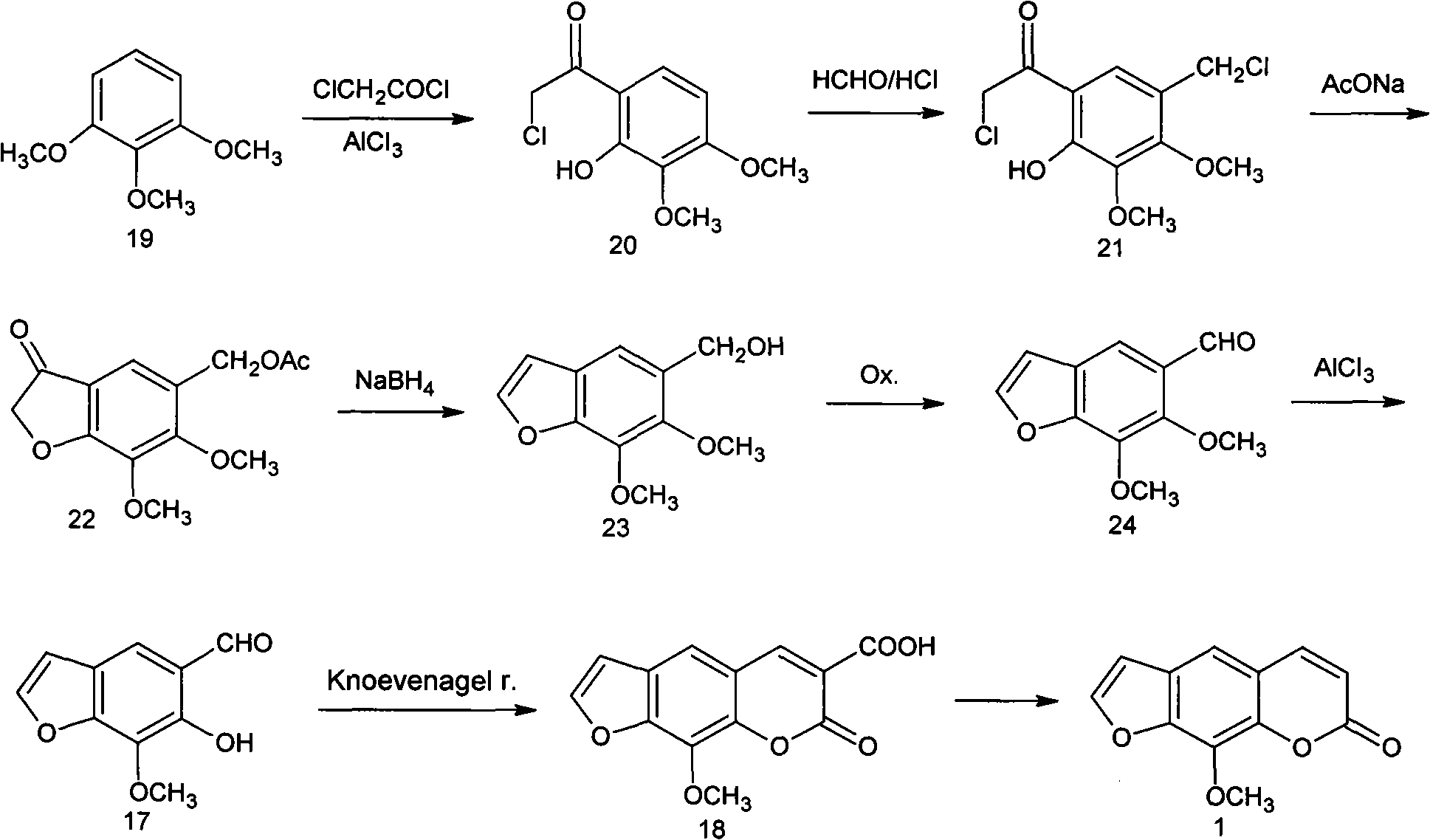
Synthesis process of methoxsalen
A synthesis process, the technology of methoxsalen, applied in the direction of organic chemistry, can solve the problem of low total yield of the process, and achieve the effect of easy purification and short synthesis process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of 2,3,4-trihydroxy-2-chloroacetophenone (3)
[0030] Mix pyrogallol 100g (0.793 mole), chloroacetic acid 78.7g (0.833 mole) and phosphorus oxychloride 60.8g (0.397 mole), react at 60-65°C for 2.5-3 hours, then add water for 1 hour. Cool down to room temperature, stand for crystallization, filter, and dry to obtain 86.3 grams (0.426 moles) of 2,3,4-trihydroxy-2-chloroacetophenone (3) as a brown powder, yield 54%, melting point 168-171°C.
Embodiment 2
[0031] Example 2 Preparation of 2,3,4-trihydroxy-2-chloroacetophenone (3)
[0032] Mix 100 grams (0.793 moles) of pyrogallol, 112 grams (1.19 moles) of chloroacetic acid and 80 grams (0.522 moles) of phosphorus oxychloride, and react at 55-62°C for 1.5-2 hours. The post-treatment method was the same as in Example 1 to obtain 122.5 g (0.549 mol) of product (3), with a yield of 69%.
Embodiment 3
[0033] Example 3 Preparation of 2,3,4-trihydroxy-2-chloroacetophenone (3)
[0034] Mix 100 grams (0.793 moles) of pyrogallol, 100 grams (1.064 moles) of chloroacetic acid and 97.4 grams (0.635 moles) of phosphorus oxychloride, and react at 50-60°C for 2-2.5 hours. The post-treatment method was the same as in Example 1 to obtain 104.3 g (0.515 mol) of product (3), with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com