Application of dioscin in preventing and treating diabetic complications
A technology of diosgenin and diabetes, which is applied in the field of medicine and can solve the problems that the role of chronic complications of diabetes has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
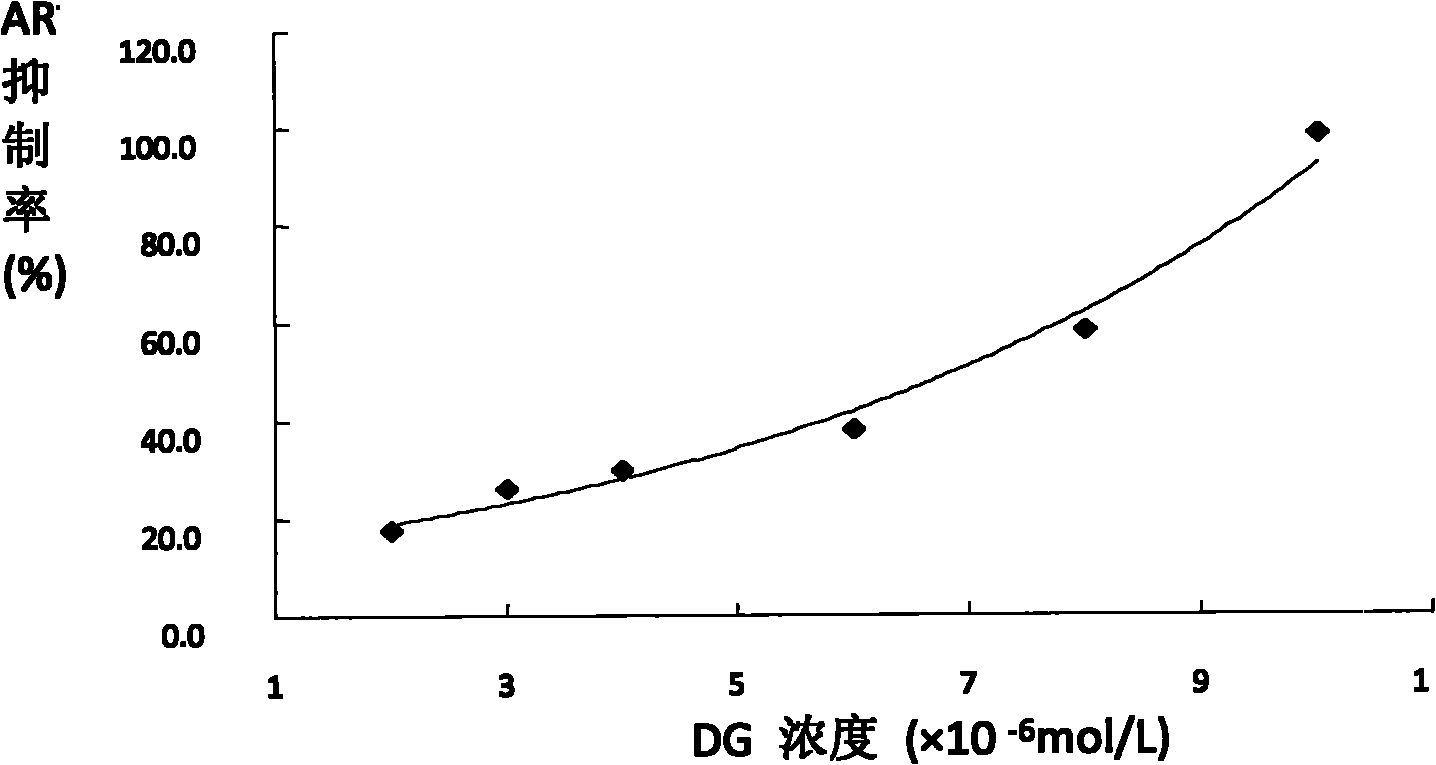
[0080] Example 1. Inhibitory effect of diosgenin on AR activity of normal rat lens
[0081] Rat lens aldose reductase (Aldose reductase, AR) extraction Take -80°C short-term frozen or fresh rat lens in EP tubes, add 400 μl pre-cooled deionized water to each lens, and ultrasonically break under ice bath (200W ×10s / time×3 times) until the entire lens is completely broken into a milky white suspension, centrifuge at 4°C (15,000g×30min), discard the precipitate, and use the supernatant for the experiment.
[0082] The inhibitory effect of diosgenin on normal rat lens AR was extracted from normal rat lens aldose reductase, with Epalrestat as positive reference, NADPH as coenzyme, DL-glyceraldehyde as substrate, blank well, NADPH control well, NADPH For metabolic wells, drug wells and drug blank wells, NADPH and NADPH-DL glyceraldehyde buffers were freshly prepared under ice bath. Firstly, a pre-experiment (blank well, NADPH well and NADPH metabolism well) was carried out, and afte...
Embodiment 2
[0084] Embodiment 2, the effect of diosgenin on the osmotic expansion of the lens of isolated cultured rats
[0085]Rat lenses were cultured in vitro from adult healthy male SD rats. Immediately after decapitation, the entire eyeball was taken out, soaked in 75% alcohol for 10 minutes, then transferred to an ultra-clean bench, and the eyeball was washed 3 times with sterilized artificial aqueous humor. Carefully remove the entire lens, roll it gently to remove the vitreous body on the surface of the lens, and then transfer it to a 24-well plate, in low sugar (1g / L), phenol red-free, serum-free DMEM medium, 37 ° C, 5% CO 2 After pre-cultivation under sterile conditions for 6 hours, 10 μl medium was taken for protein quantification by the Coomassie Brilliant Blue method, and the lens that was completely transparent and the protein content of the medium was less than 100 μg / ml was selected as the undamaged lens during the operation for subsequent experiments. The effect of diosge...
Embodiment 3
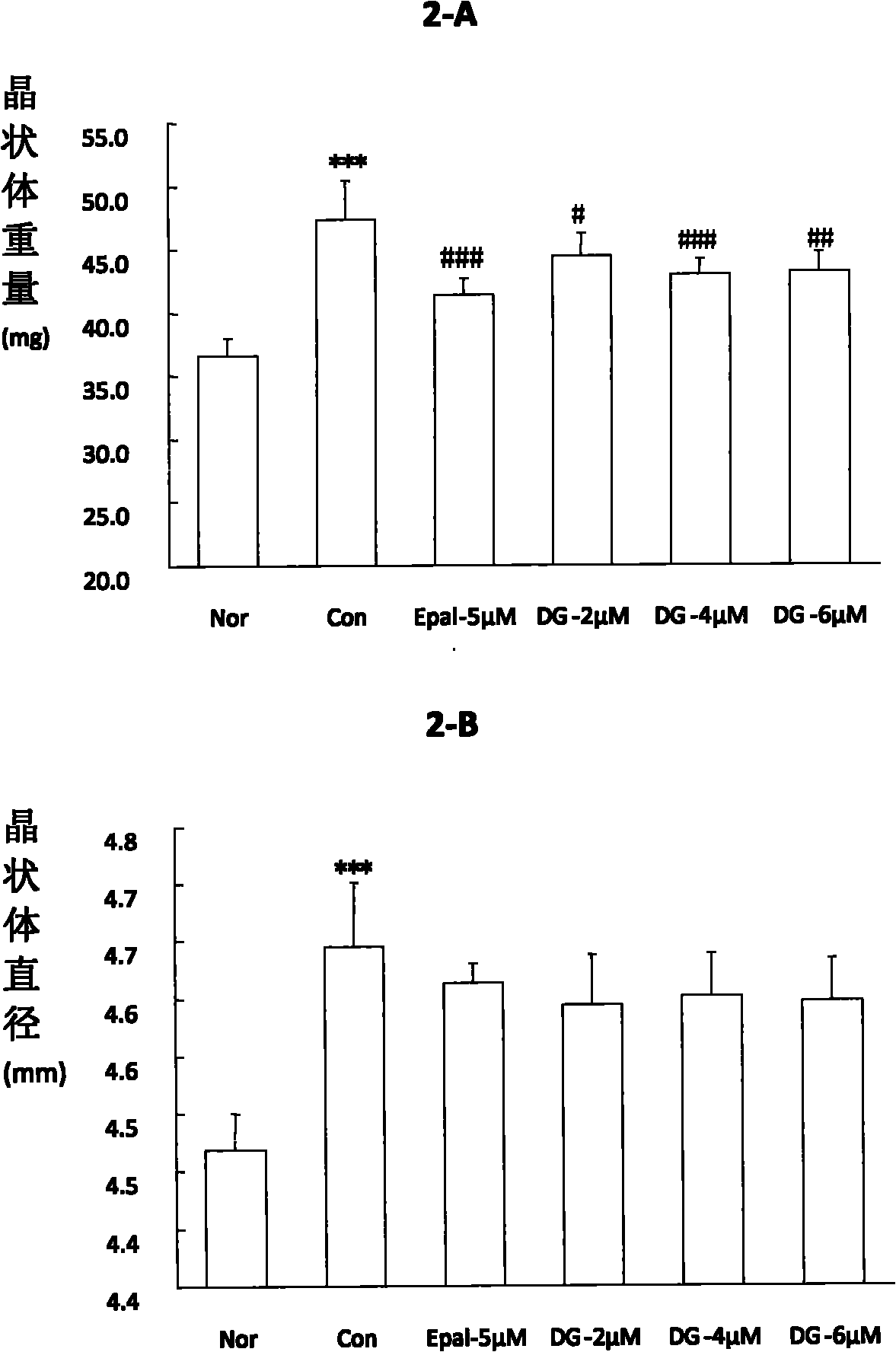
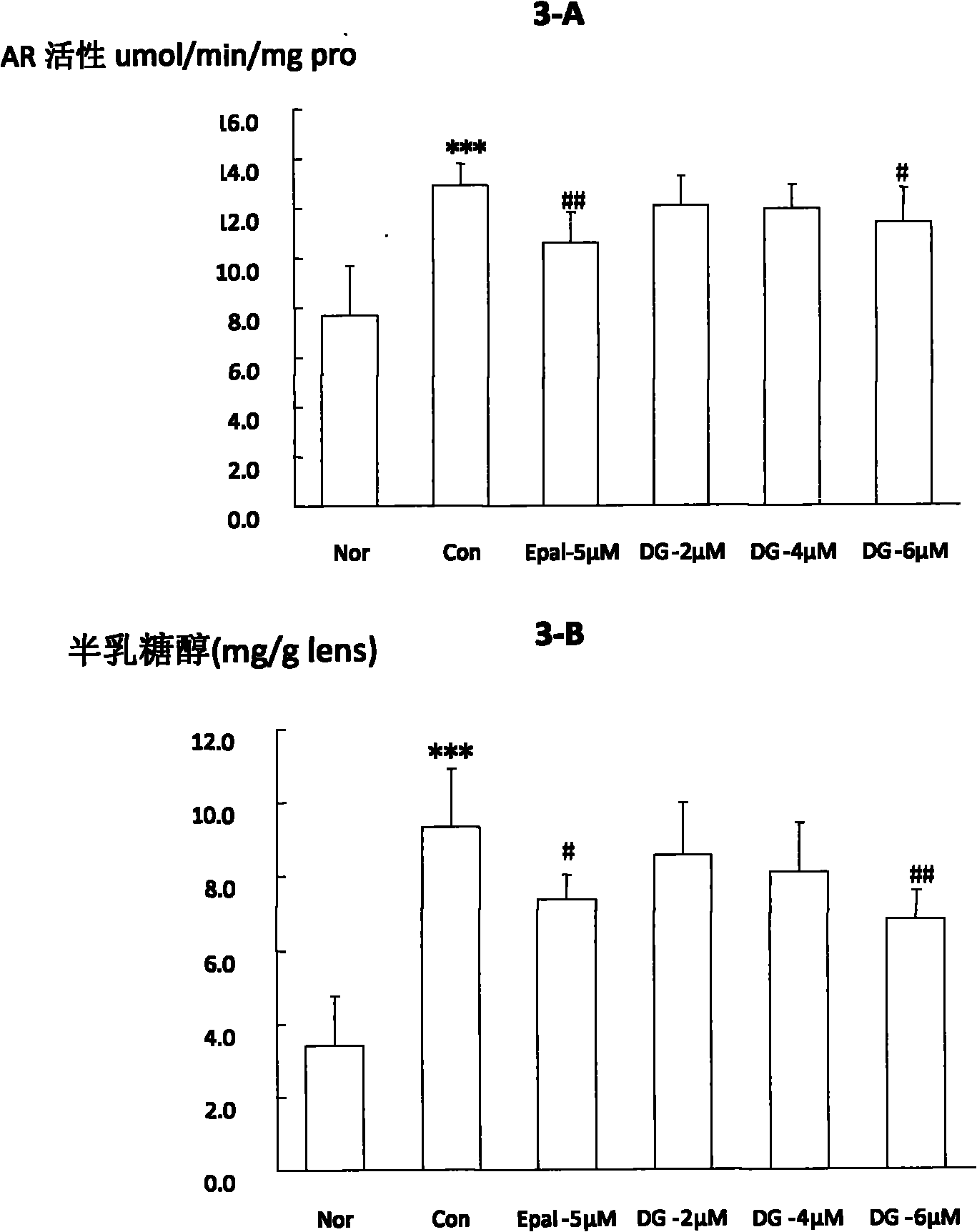
[0094] Embodiment 3, the impact of oral administration of diosgenin on cataract in galactose rats
[0095] experimental design
[0096] Set normal group, galactose model group and different concentrations of dioscin groups (DG 100, 200, 0.1%), n=10. Oral administration was adopted in the experiment. Rats in DG(100, 200) groups were intragastrically administered at doses of 100 and 200 mg / kg respectively, and rats in DG-0.1% group continued to drink 0.1% diosgenin solution. Rats in the normal group and the model group were given the same amount of normal drinking water by intragastric administration for 15 days.
[0097] Experimental operation
[0098] 20-day-old normal male Wistar pups with completely transparent lenses were randomly divided into 5 groups and administered orally. From 22 days old, the rats in the model group and the drug-administered group were given 12.5% galactose solution from the 1st to the 7th day For feeding, 10% galactose solution was given on the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com