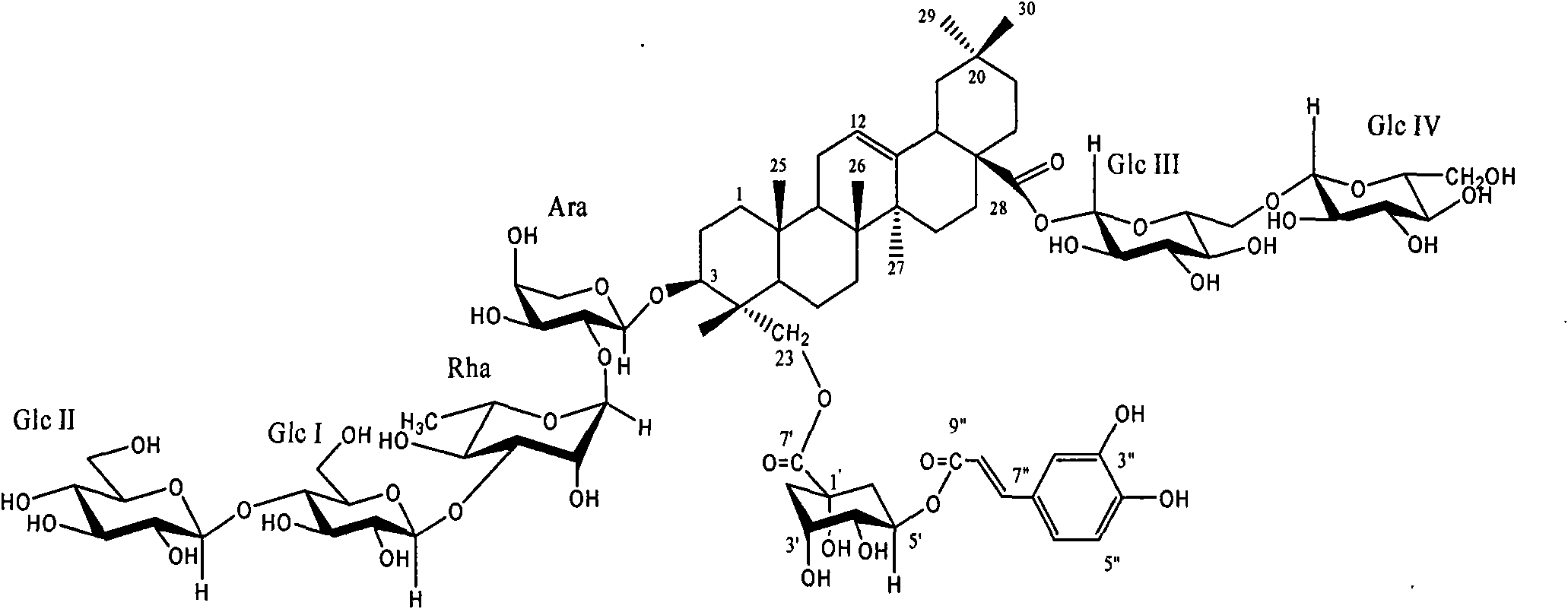
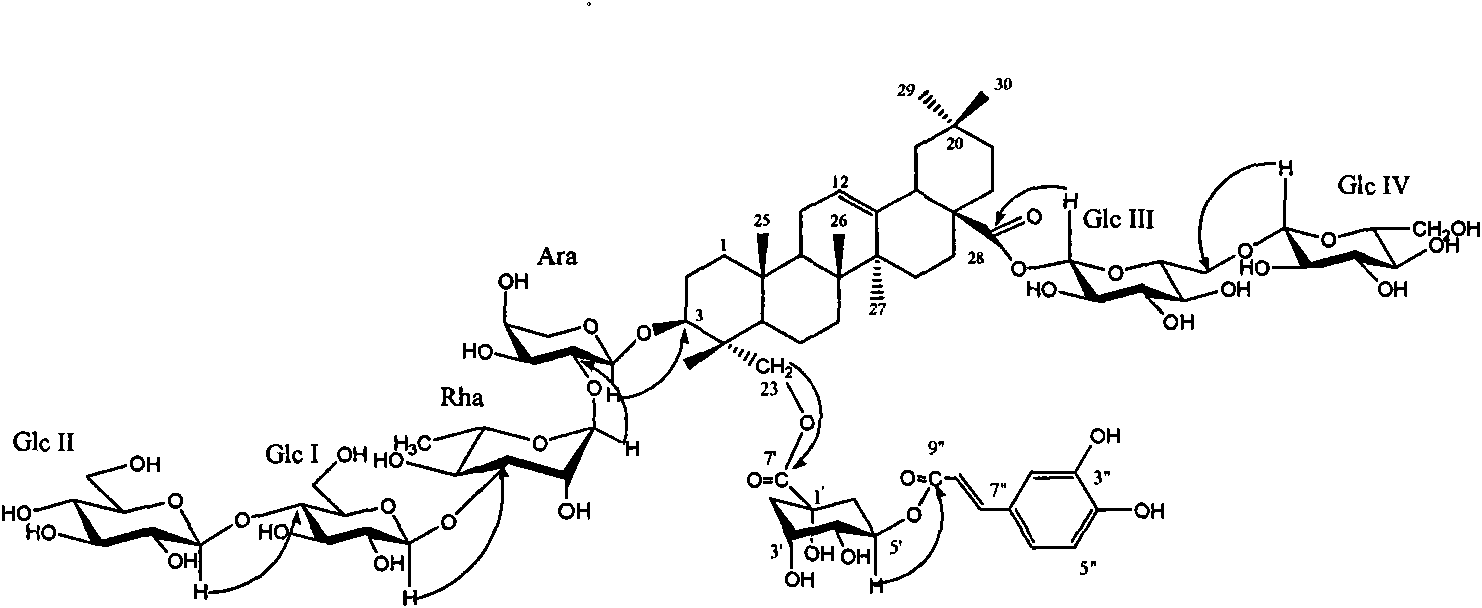
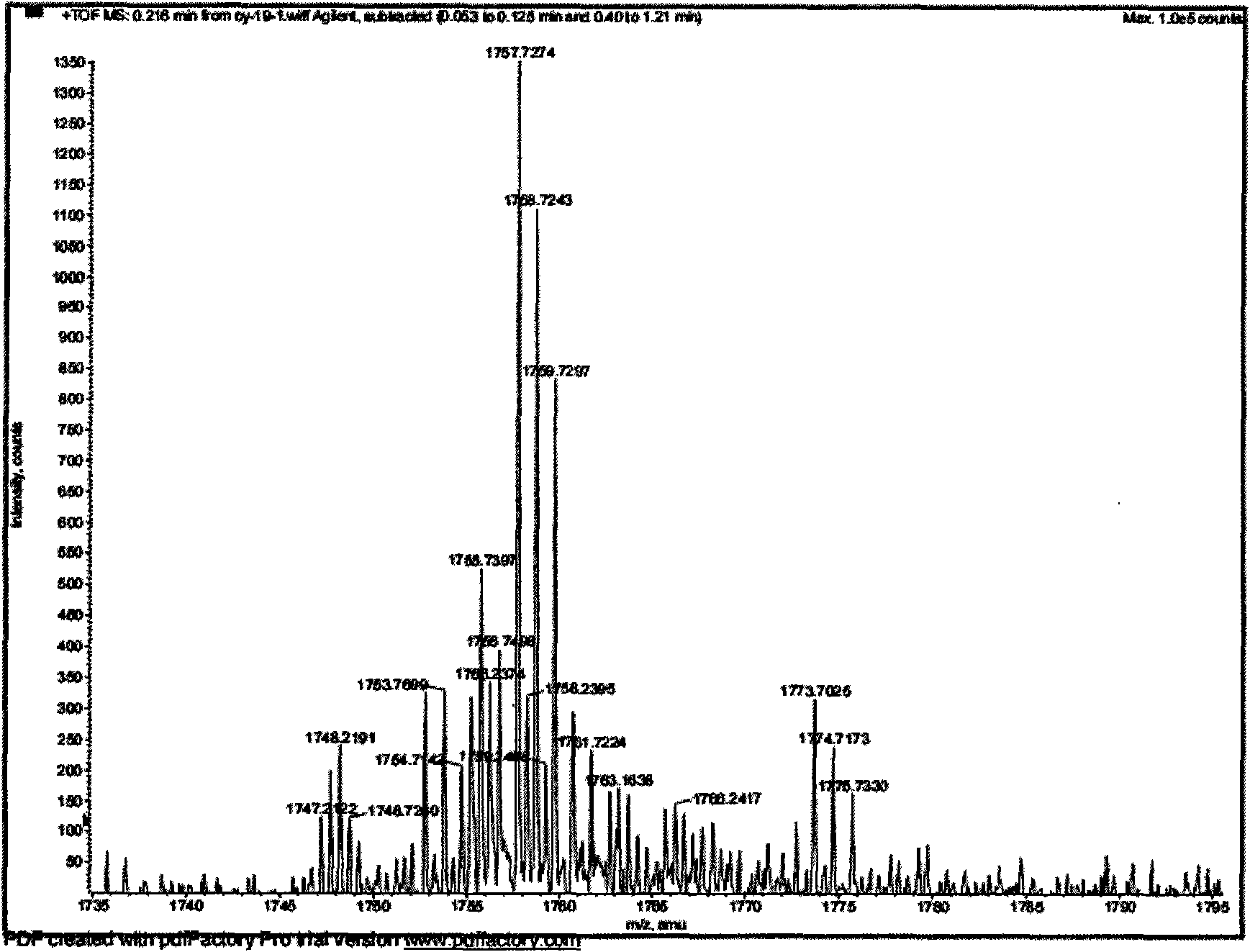
New honeysuckle chlorogenic acid ester saponin as well as preparation method and use thereof
A technology of chlorogenic ester saponins and compounds, applied in the field of natural medicinal chemistry, can solve the problem that COX-2 inhibitors cannot be used in clinical cancer chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Isolation and identification of the compound Lonimacranthoide I from Lonicera cinerea
[0022] 10 kg of dried flower buds of Lonicerae pilosula were extracted with 100 kg of 95% (volume ratio) ethanol-water solution under reflux for 3 times, each time for 2 hours, and concentrated until there was no alcohol smell to obtain an extract (dry weight 1.7 kg); the obtained extract was added 10 times volume of water dissolved, filtered through filter paper to remove water-insoluble matter, the filtrate was adsorbed with macroporous resin HP-20, then eluted with water, 20%, 70% ethanol solution, combined with 70% ethanol solution eluate, concentrated to obtain gray felt Total saponins of Lonicerae pubescens 860g. The obtained total saponins of Lonicera tomentosa were subjected to silica gel column chromatography, and the eluent was chloroform-methanol-water (17:3:0.2→4:1:0.1→7:3:0.5→3:3:0.5), methanol Wherein chloroform-methanol-water (3: 3: 0.5) part carries out re...
Embodiment 2
[0031] Embodiment 2, water extraction method prepares Lonimacranthoide I from gray felt wool honeysuckle
[0032] 1 kg of dried flower buds of Lonicerae pilosula, heated and extracted three times with water, the water consumption was 10 liters, the extraction time was 2 hours, the extraction temperature was 100°C, the extract was absorbed by macroporous resin D101, and then washed with water, 15%, 75% ethanol Take off, and 75% ethanol eluent recovers solvent under reduced pressure to obtain 87g of total saponins of Lonicera tomentosa. The obtained total saponins of Lonicera tomentosa were subjected to silica gel column chromatography, and the mobile phase was chloroform-methanol-water (17:3:0.2→4:1:0.1→7:3:0.5→3:3:0.5), methanol; Among them, chloroform-methanol-water (3:3:0.5) was subjected to repeated reverse phase silica gel C-8 column chromatography (eluent was 30% ethanol-water solution) separation and gel column Sephdex LH-20 chromatography purification (elution The solv...
Embodiment 3
[0033] Embodiment 3, prepare Lonimacranthoide I from gray felt wool honeysuckle with methanol cold soaking extraction method
[0034] 1Kg of dried flower buds of Lonicerae pilosula, extracted three times by cold immersion in methanol, the amount of methanol used was 20 liters, the extraction time was 20 days, the extraction temperature was room temperature, and the extract was concentrated to an alcohol-free extract (dry weight was 120g); Add 10 times the volume of water to dissolve the extract, filter paper to remove water-insoluble matter, absorb the filtrate with macroporous resin AB-8, then elute with water, 10%, 80% ethanol solution, and combine the 80% ethanol solution eluent , and concentrated to obtain 81g of total saponins of Lonicerae cinerea. Gained total saponins of Lonicera tomentosa are subjected to column chromatography (silica gel column chromatography: chloroform-methanol-water gradient elution system, RP-C18 column chromatography: water-methanol system, gel c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com