Continuous process to produce hexafluoroisopropanol
A technology of hexafluoroisopropanol and hexafluoroacetone, which is applied in the continuous field of preparing hexafluoroisopropanol, and can solve the problems of shortening catalyst life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
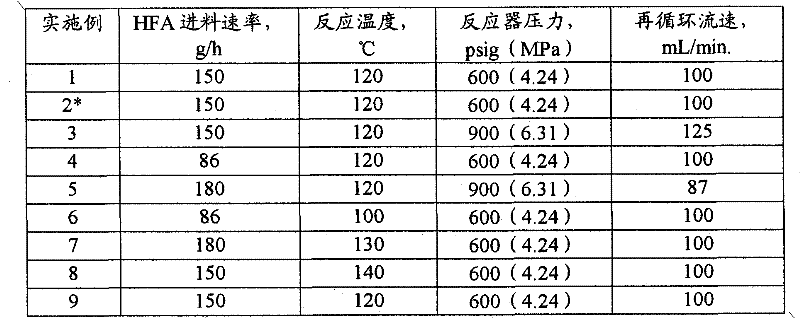
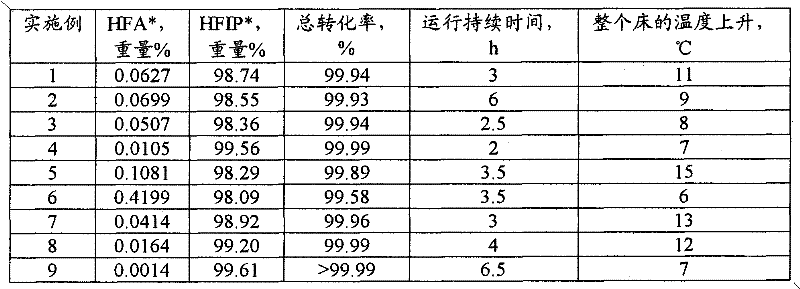
[0074] The steps for start-up and continuous operation of the preferred method used in the following examples are described. The examples provide the process variables used.
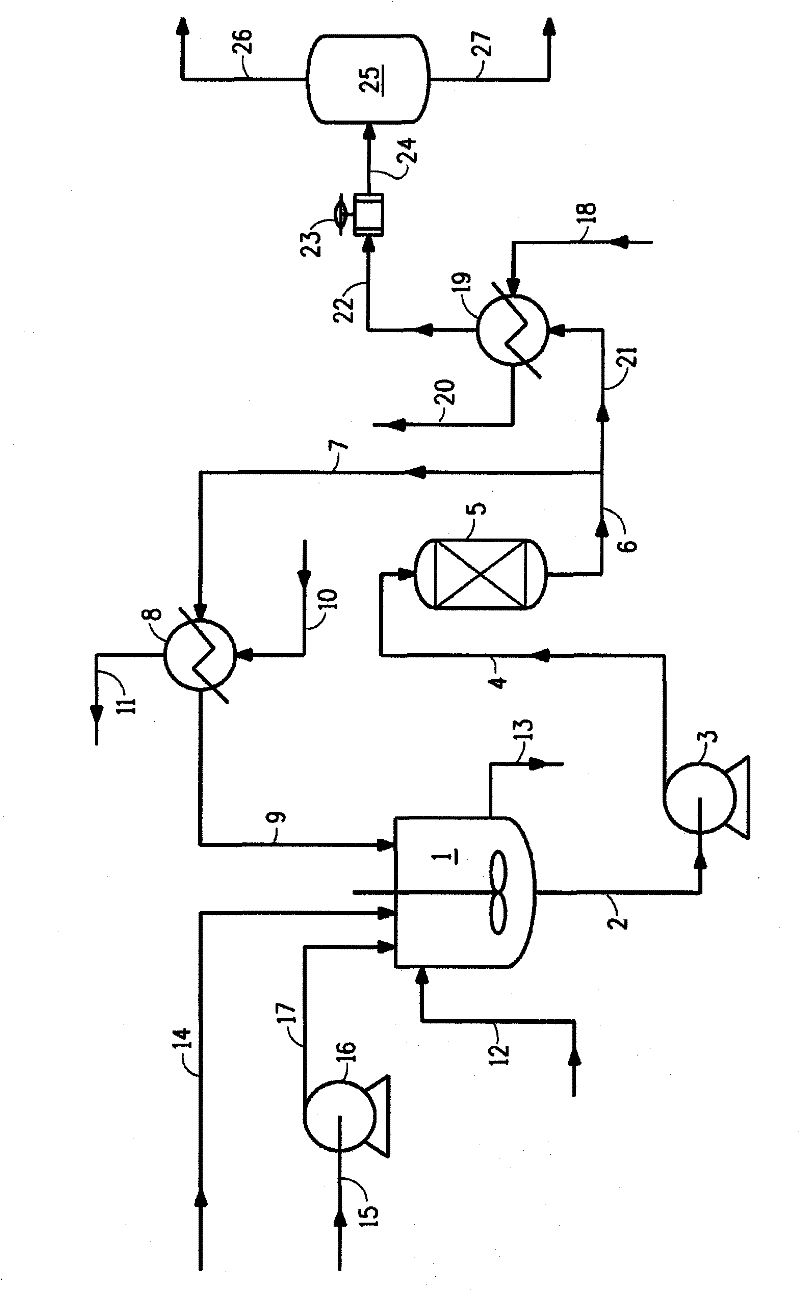
[0075] Said embodiment works with figure 1 performed in a similar reaction system. The mixing equipment 1 is a 1-liter 316 stainless steel autoclave equipped with a stirrer and a heating device, and has a design pressure of 2000 psig (13.9 MPa). HFA was fed to the autoclave with an Isco syringe pump 16, model 1000D. Pump 3 is a Lewa Ecoflow 319 stainless steel diaphragm pump with a design pressure of 1500 psig (10.4 MPa). Reactor 5 was a 16 x 1 inch (40.6 x 2.54 cm) 316 stainless steel Schedule 80 tube. The product was collected in a 17 x 4 inch (43.2 x 10.2 cm) 304 stainless steel gas / liquid separator 25, which had a capacity of 2.25 L. The catalysts used were Engelhard 1 / 16 inch (1.6 mm) 3F Ni-3288E trilobe catalyst (41.5 g) and Johnson Matthey HTC NI500RP trilobe catalyst (39.2 g).
[0076] In t...
Embodiment 2
[0080] Example 2 used the same reactor configuration as above except that the reactor was used in a downflow configuration instead of an upflow configuration. Reactor feed enters at the top of the bed and product exits at the bottom of the bed.
Embodiment 9
[0081] Example 9 used essentially the same upflow reactor configuration, except that a 6 inch (15.2 cm) Johnson Matthey HTC Ni500 1.2RP trilobe catalyst was used, having a total weight of 39.16 g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com