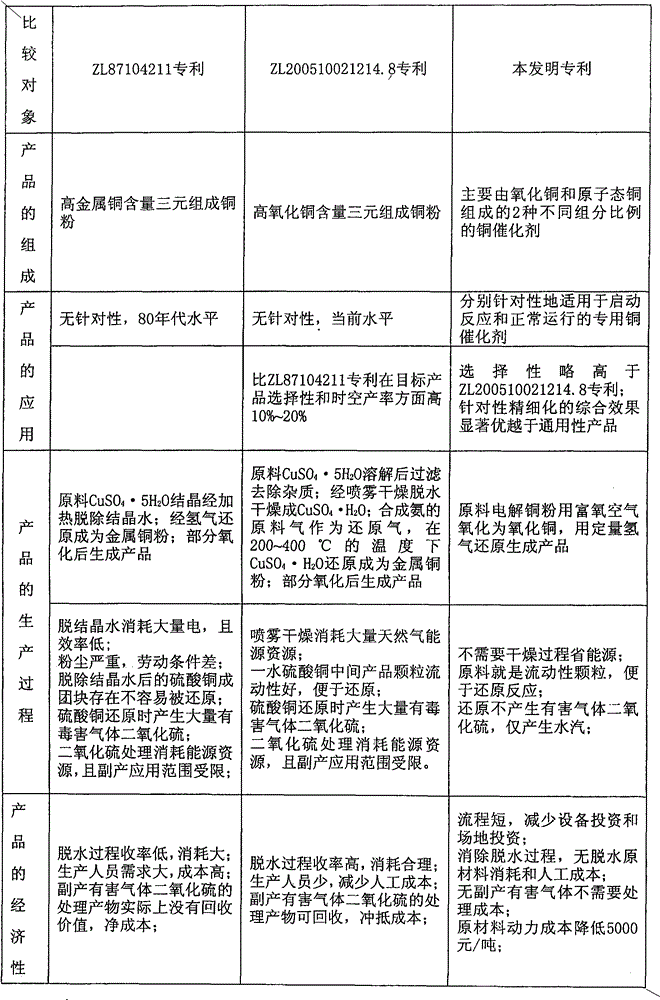
Two types of catalysts for synthesizing methyl chlorosilane and preparation method thereof
A methylchlorosilane, copper catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of long dehydration time, large energy consumption, use of problems such as narrow field, to achieve the effect of simple production process, low production cost and best application effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The copper catalyst 2 used in the initial start-up reaction of the methyl chlorosilane synthesis reaction is made of metal copper powder as a raw material, after oxidation to remove surface impurities, deep oxidation to change the material structure, and partial reduction to adjust the composition of the product. Its composition and weight percentage are as follows:
[0038] Metal Copper 58.42% Cuprous Oxide 29.18% Copper Oxide 12.40%
[0039] The metallic copper is electrolytic copper powder with an average particle size of 300 mesh.
[0040] A. Oxidation to remove surface impurities: put electrolytic copper powder in a fluidized bed reactor, pass through oxygen-enriched air with an oxygen content of 20-25% (volume), and oxidize at 120-150°C for 15-30 minutes to remove the surface Protective organic impurities;
[0041] B. Deep oxidation to change the material structure: continue to pass in oxygen-enriched air with an oxygen content of 20-30% (volume), and oxidize at...
Embodiment 2
[0047] The copper catalyst 1 used for the normal reaction after the start of the synthesis reaction of methyl chlorosilane is made of metal copper powder as raw material, which is oxidized to remove surface impurities, deep oxidized to change the material structure, and partially reduced to adjust the composition of the product. Its composition and weight percentage are as follows:
[0048] Metallic copper 18.80%, cuprous oxide 25.66%, copper oxide 55.54%
[0049] The metallic copper is electrolytic copper powder with a particle size of 300 mesh.
[0050] A. Oxidation to remove surface impurities: put electrolytic copper powder in a fluidized bed reactor, pass through oxygen-enriched air with an oxygen content of 20-25% (volume), and oxidize at 120-150°C for 15-30 minutes to remove the surface Protective organic impurities;
[0051] B. Deep oxidation to change the material structure: continue to pass in oxygen-enriched air with an oxygen content of 20-30% (volume), and oxidi...
Embodiment 3
[0057] The copper catalyst 1 used for the normal reaction after the start of the synthesis reaction of methyl chlorosilane is made of metal copper powder as raw material, which is oxidized to remove surface impurities, deep oxidized to change the material structure, and partially reduced to adjust the composition of the product. Its composition and weight percentage are as follows:
[0058] Metallic copper 25.55%, cuprous oxide 29.88%, copper oxide 44.57%
[0059] The metal copper powder is electrolytic copper powder with a particle size of 200 mesh.
[0060] A. Oxidation to remove surface impurities: put electrolytic copper powder in a fluidized bed reactor, pass through oxygen-enriched air with an oxygen content of 20-25% (volume), and oxidize at 120-150°C for 15-30 minutes to remove the surface Protective organic impurities;
[0061] B. Deep oxidation to change the material structure: continue to pass in oxygen-enriched air with an oxygen content of 20-35% (volume), and o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com