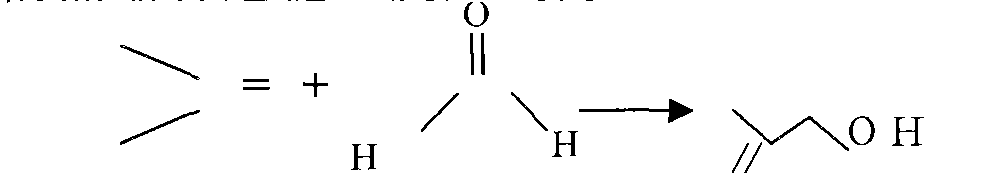
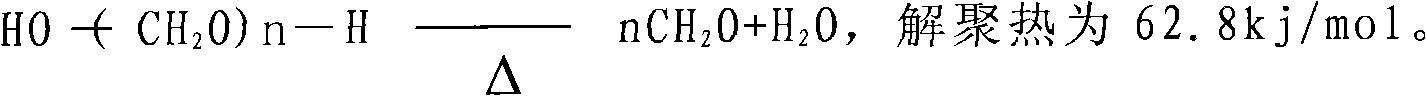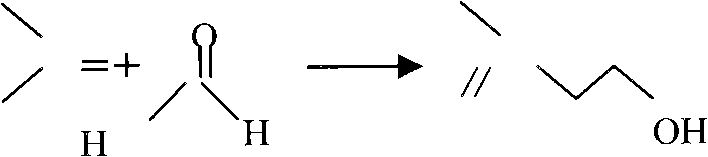Method for gas-phase solvent-free catalysis-free synthesis of 3-methyl-3-butenyl-1-alcohol
A solvent-free, butenyl-based technology is applied in the preparation field of solvent-free and catalytic synthesis of 3-methyl-3-butenyl-1-ol, which can solve the problems of low yield and the like, and achieves reduction of energy consumption and savings Operation steps, the effect of reducing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1:. The depolymerization of paraformaldehyde
[0028] In order to reduce side reactions and facilitate the refining of products, 3g of industrial paraformaldehyde and 30g of isobutylene are pulped first. After the 280ml stirred autoclave was replaced with N2, the slurry was pressed into the autoclave, the temperature was raised, the temperature and pressure were observed, and the gas composition was detected, and the depolymerization temperature, pressure and residence time of paraformaldehyde were calculated. The present invention controls the temperature to be 120-200° C., the depolymerization time to be 10-60 minutes, and the pressure to be 9.0-15.0 Mpag.
[0029] Paraformaldehyde HO-(CH 2 O) n-H, where n is generally 8 to 100, and formaldehyde is 90-99%. We use lower values of n. Formaldehyde purity is 90-94%. Instead of using α, β, γ, δ and ε type paraformaldehyde to reduce the difficulty of depolymerization. Paraformaldehyde and isobutylene (liqu...
Embodiment 2
[0032] Example 2: Condensation reaction of isobutylene and formaldehyde prins to generate isopentenol (3.3.1).
[0033] The above-mentioned isobutene, formaldehyde, and the isobutene calculated according to the ratio of isobutene: formaldehyde = 8 ~ 15 mol ratio are heated and vaporized with high-pressure nitrogen, and then pressed into a plug flow reactor immersed in a hot oil bath, and the temperature is controlled at 200 ~ 300°C, pressure 9.0-15.0Mpag, residence time 1-3h; during this period, measure the composition of prenol (3, 3, 1), formaldehyde and isobutene in the reactor every 10 minutes until the residence time is reached, and then react The product is placed in a dry ice bath at -70°C to condense it completely, and the liquid phase composition is measured. The product isopentenol (3, 3, 1) isobutene and moisture are deducted, and the concentration is greater than 95wt%. The isobutene is separated and recycled. The product yield was 90 wt% based on isobutene and 97 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com