Polypeptide combined with various amyloid protein monomers simultaneously and application thereof
A technology of amyloid and monomers, applied in the direction of immunoglobulin, medical preparations containing active ingredients, peptides, etc., can solve the problems of patient death and achieve the effect of eliminating side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A polypeptide (PEMX for short) that binds amyloid monomers at the same time, its amino acid sequence is: Cys-Trp-His-Thr-Asp-Thr-Arg-Ser-Cys, or the above amino acid sequence has been substituted, deleted, Or add one or several amino acids and have the function of inhibiting the formation of amyloid oligomers. The invention can effectively stimulate the body to produce specific antibodies combined with multiple amyloid amyloid transition state monomers, and the antibodies can inhibit amyloid aggregation and cytotoxicity.
[0027]In a specific embodiment of the present invention, the N-terminal of the polypeptide of the present invention is modified with PEG2000 (referred to as PEG-PEMX) (sequence: PEG-Cys-Trp-His-Thr-Asp-Thr-Arg-Ser- Cys), replacement of aspartic acid with alanine (referred to as PEMX-D / A) (sequence: Cys-Trp-His-Thr-Ala-Thr-Arg-Ser-Cys), deletion of tryptophan (referred to as for: PEMX-?W) (sequence: Cys-His-Thr-Asp-Thr-Arg-Ser-Cys) or inserted glycine...
Embodiment 2
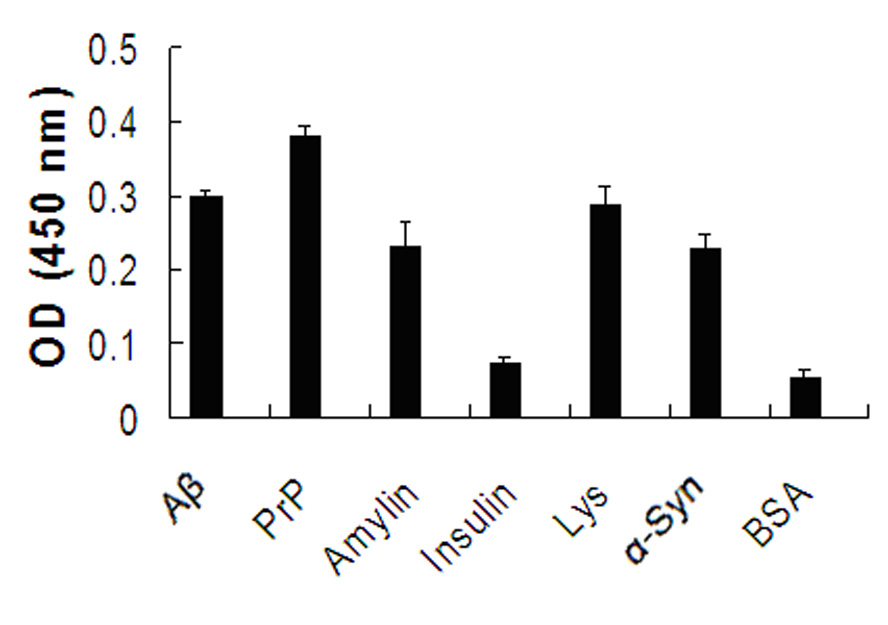
[0038] Example 2: PEMX can bind to various amyloid monomers
[0039] 100 μl Ab42, PrP, amylin, a-synuclein (American Peptide Company, USA), insulin, lysozyme (Sigma-Aldrich, USA) monomers (1 μg / well) were respectively coated on 96-well ELISA microplates , BSA as negative control, placed at 37°C for 2 h, blocked with 3% BSA at 37°C for 2 h, 200 μL / well. The plate was washed 3 times with PBS, 100 μl of PEMX solution with histidine tag was added, incubated at room temperature for 1 h, and washed three times with PBS containing 0.1% Tween-20. Add 100 μl of HRP-linked histidine tag-recognizing antibody (9E10) (1:3000) to each well, incubate at room temperature for 1 h, and wash 3 times with PBS containing 0.1% Tween-20. Add 100 μL of substrate solution TMB to each well, place at 37°C for 20 min, then add 50 μl of 1 mmol / L sulfuric acid to each well to terminate the reaction, and measure the OD value (wavelength: 450 nm) on an enzyme-linked immunosorbent detector ( figure 1 ). fi...
Embodiment 3
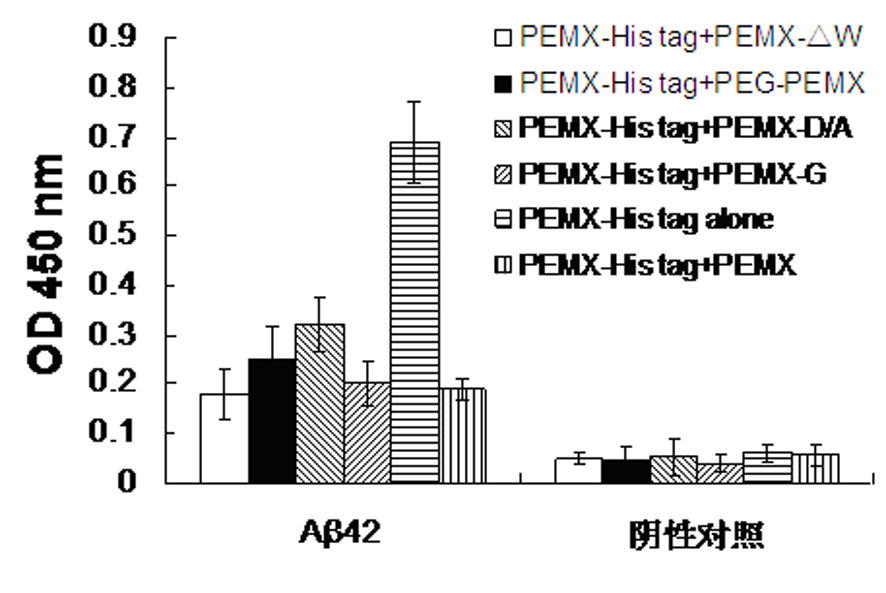
[0040] Example 3: PEMX with substituted, deleted or inserted amino acids still binds to Aβ42
[0041] The N-terminus of the polypeptide PEMX was modified with PEG2000 (referred to as PEG-PEMX) (sequence: PEG-Cys-Trp-His-Thr-Asp-Thr-Arg-Ser-Cys), and aspartic acid was replaced with alanine amino acid (abbreviated as PEMX-D / A) (sequence: Cys-Trp-His-Thr-Ala-Thr-Arg-Ser-Cys), delete tryptophan (abbreviated as: PEMX-?W) (sequence: : Cys-His-Thr-Asp-Thr-Arg-Ser-Cys) or inserted glycine (referred to as: PEMX-G) (sequence: Cys-Trp-His-Thr-Asp-Thr-Arg-Gly-Ser- Cys). In order to be able to detect the binding of the modified polypeptides to Aβ42, these polypeptides compete with unmodified PEMX with a tag to bind to Aβ42, and then ELISA is used to detect the binding of the latter to Aβ42. Add 100 ml of a mixture of 1 mg unmodified tagged PEMX and 10 mg modified PEMX to a 96-well ELISA plate coated with Aβ42 monomer, and then use ELISA to detect the binding of tagged PEMX to Aβ42 , mea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com