Method for removing iron in leaching liquid of lateritic nickel ore by oxidation
A technology for oxidizing iron and laterite nickel ore is applied in the field of hydrometallurgy, which can solve the problems of high iron removal cost, high manganese ion content, influence on treatment, etc., and achieves the effect of good iron removal effect and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
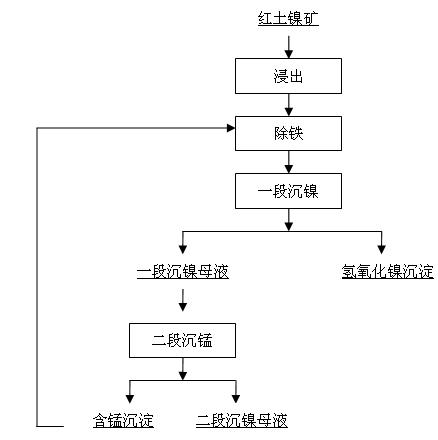
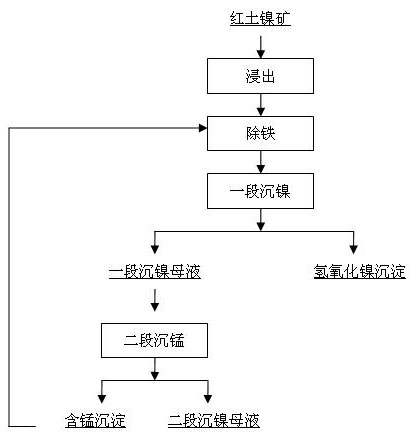
[0019] The leach solution contains Fe 2+ 1.7g / l, containing Mn 2+ It is 0.97g / l. The pH of the first-stage nickel precipitation is 8.3, the reaction temperature is 70 degrees Celsius, and the reaction time is 3 hours; the pH of the second-stage manganese precipitation is 9.8, the temperature is 70 degrees Celsius, and the reaction time is 2 hours. The second-stage manganese precipitation slag contains 10% manganese. The second-stage manganese precipitation slag is added to the iron removal section. The actual amount added is 3 times the theoretical calculation amount based on the reaction equation. The reaction temperature is 60 degrees Celsius and the pH value is 0.5 to oxidize ferrous ions into ferric ions. Then increase the pH to 1.7-5.0, the reaction time is 3 hours, the ferric iron ion is hydrolyzed into ferric hydroxide or jarosite precipitation, thereby removing iron, and Fe in the liquid after iron removal 2+ The concentration is <0.002g / l.
Embodiment 2
[0021] The leach solution contains Fe 2+ 1.5g / l, containing Mn 2+ It is 0.82g / l. The pH of the first-stage nickel precipitation is 8.5, the temperature is 62 degrees Celsius, and the reaction time is 1 hour; the pH of the second-stage manganese precipitation is 9.6, the temperature is 62 degrees Celsius, the reaction time is 2.5 hours, and the second-stage manganese precipitation slag contains 6% manganese. The second-stage manganese precipitation slag is added to the iron removal section. The actual amount added is 4 times the theoretical calculation amount based on the reaction equation. The reaction temperature is 65 degrees Celsius and the pH value is 1.0 to oxidize ferrous ions into ferric ions. Then increase the pH to 3.6-4.0, and the reaction time is 4 hours. The ferric ion is hydrolyzed into ferric hydroxide or jarosite precipitation, thereby removing iron, and Fe in the solution after iron removal 2+ The concentration is <0.002g / l.
Embodiment 3
[0023] The leach solution contains Fe 2+ 1.1g / l, containing Mn 2+ It is 0.63g / l. The pH of the first stage of nickel precipitation is 8.3, the temperature is 50 degrees Celsius, and the reaction time is 1.5 hours; the pH of the second stage of manganese precipitation is 9.4, the temperature is 50 degrees Celsius, and the reaction time is 3 hours. The second stage of manganese precipitation slag contains 4% manganese. The second-stage manganese precipitation slag is added to the iron removal section. The actual amount added is 5 times the theoretically calculated amount according to the reaction equation. The reaction temperature is 70 degrees Celsius and the pH value is 1.4 to oxidize ferrous ions into ferric ions. Then increase the pH to 3.6-4.7, the reaction time is 5 hours, the ferric ions are hydrolyzed into ferric hydroxide or jarosite precipitation, thereby removing iron, and Fe in the solution after iron removal 2+ The concentration is <0.002g / l.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com