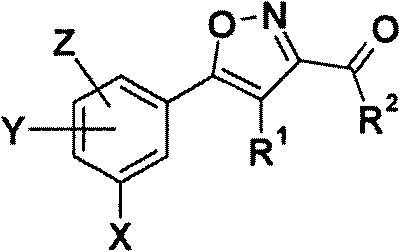
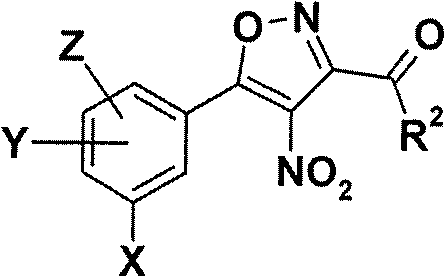
5-phenyl-isoxazole-3-carboxamides modulating HSP90 with antitumoral activities
A technology of isoxazole and formyl ethylamine, which can be used in antineoplastic drugs, organic active ingredients, and resistance to vector-borne diseases, etc., and can solve the safety and stability problems of Hsp90 inhibitors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 4-Acetamido-5-(5-chloro-2,4-dihydroxy-phenyl)isoxazole-3-formylethylamine SST0072AA1
[0136] Step i: 1-(5-Chloro-2,4-dihydroxyphenyl)-ethanone
[0137] Acetic acid (17.5ml) was added dropwise to 4-chlorobenzene-1,3-diol (20g, 0.138mol) in BF under nitrogen atmosphere 3 .OEt 2 (100ml). The reaction mixture was stirred at 90°C for 3.5 hours and then cooled to room temperature, allowing a solid to precipitate. This mixture was poured into 10% w / v aqueous sodium acetate (350ml). The mixture was then stirred vigorously for 2.5 hours to afford a light brown solid which was filtered, washed with water, and air dried overnight to afford the title compound 1-(5-chloro-2,4-dihydroxyphenyl)-ethanone ( 11.3 g, 44%).
[0138] 1 H NMR (200MHz CDCl 3 ), δ: 2.56 (s, 3H), 6.11 (s, 1H), 6.59 (s, 1H), 7.70 (s, 1H), 12.48 (s, 1H).
[0139] Step ii: 1-[2,4-bis(benzyloxy)-5-chlorophenyl]ethanone
[0140] Benzyl bromide (17.5ml, 0.147mol) was added to 1-(5-chloro-2,4-dihydroxyphenyl...
Embodiment 2
[0170] 5-(5-Chloro-2,4-dihydroxy-phenyl)-4-(4-methoxy-benzoylamino)-isoxazole-3-formylethylamine SST0081AA1
[0171] Step viii: 5-(2,4-Bis-benzyloxy-5-chloro-phenyl)-4-(4-methoxy-benzoylamino)-isoxazole-3-formylethyl amine
[0172] 1 H NMR (200MHz CDCl 3 ), δ: 1.28(t, J=7.2Hz, 3H), 3.45-3.54(m, 2H), 3.84(s, 3H), 4.85(s, 2H), 5.11(s, 2H), 6.57(s, 1H), 6.83(d, J=9.0Hz, 2H), 6.95(br, 1H), 7.08-7.13(m, 2H), 7.21-7.29(m, 2H), 7.35-7.43(m, 6H), 7.55 (d, J = 9.0 Hz, 2H), 7.75 (s, 1H), 9.47 (s, 1H).
[0173] [M+H] + 612.1 / 613.3
[0174] Step ix: 5-(5-Chloro-2,4-dihydroxy-phenyl)-4-(4-methoxy-benzoylamino)-isoxazole-3-formylethylamine
[0175] 1 H NMR (400MHz DMSO), δ: 1.06(t, J=6.8Hz, 3H), 3.19-3.24(m, 3H), 3.82(s, 3H), 6.64(s, 1H), 7.04(d, J= 8.8Hz, 2H), 7.43(s, 1H), 7.87(d, Hz, 2H), 8.71(t, J=5.6Hz, 1H), 9.62(s, 1H), 10.48(br, 1H), 10.68( s, 1H).
[0176] 13C NMR (100 MHz DMSO), δ: 14.4, 33.5, 55.3, 103.8, 106.2, 110.4, 113.6, 125.7, 129.4, 155.3, 155.5, 155.7, 158.5, 16...
Embodiment 3
[0178] 5-(5-Chloro-2,4-dihydroxy-phenyl)-4-(3,4-dimethoxy-benzoylamino)-isoxazole-3-formylethylamine SST0100AA1
[0179] Step viii: 5-(2,4-Bis-benzyloxy-5-chloro-phenyl)-4-(3,4-dimethoxy-benzoylamino)-isoxazole-3-carba Acylethylamine
[0180] 1 H NMR (200MHz CDCl 3 ), δ: 1.27(t, J=7.2Hz, 3H), 3.46-3.54(m, 2H), 3.84(s, 3H), 3.92(s, 3H), 4.85(s, 2H), 5.10(s, 2H), 6.57(s, 1H), 6.74(d, J=8.6Hz, 1H), 6.94(br, 1H), 7.05-7.12(m, 2H), 7.22-7.25(m, 2H), 7.32-7.40 (m, 8H), 7.75 (s, 1H), 9.56 (s 1H).
[0181] Step ix: 5-(5-Chloro-2,4-dihydroxy-phenyl)-4-(3,4-dimethoxy-benzoylamino)-isoxazole-3-formylethylamine
[0182] 1 H NMR (400MHz DMSO), δ: 1.06(t, J=6.8Hz, 3H), 3.18-3.22(m, 3H), 3.79(s, 3H), 3.81(s, 3H), 6.63(s, 1H) , 7.05(d, J=8.8Hz, 1H), 7.41(s, 1H), 7.43(d, J=1.6Hz, 1H), 7.52(dd, J=8.8, J=1.6Hz, 1H), 8.69( t, J = 6.0 Hz, 1H), 9.59 (s, 1H), 10.44 (br, 1H), 10.66 (s, 1H).
[0183] 13 C NMR (100 MHz DMSO), δ: 14.4, 33.5, 55.5, 55.6, 103.8, 106.2, 110.7, 110.9, 113.6, 120.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com