Preparation method for improving quality of medium molecular weight hydroxyethyl starch
A technology of hydroxyethyl starch and relative molecular weight, which is applied in the field of medicine and can solve problems such as medical accidents, potential safety hazards in clinical use, and adventitia shedding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
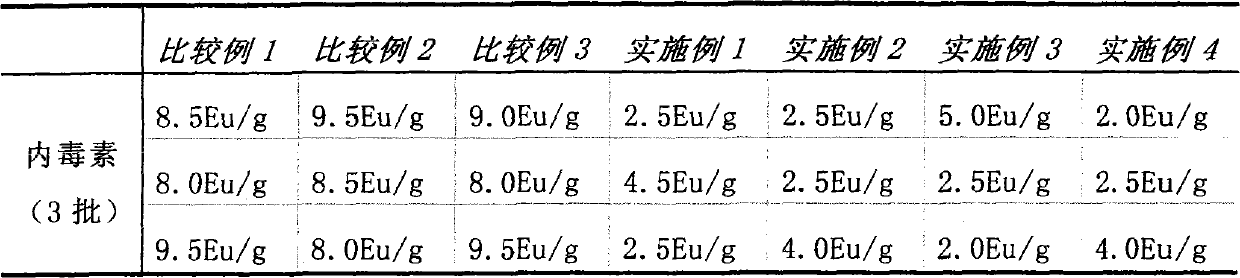
Examples
Embodiment Construction
[0008] The preferred embodiments of the present invention will be described below in conjunction with the accompanying drawings. It should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
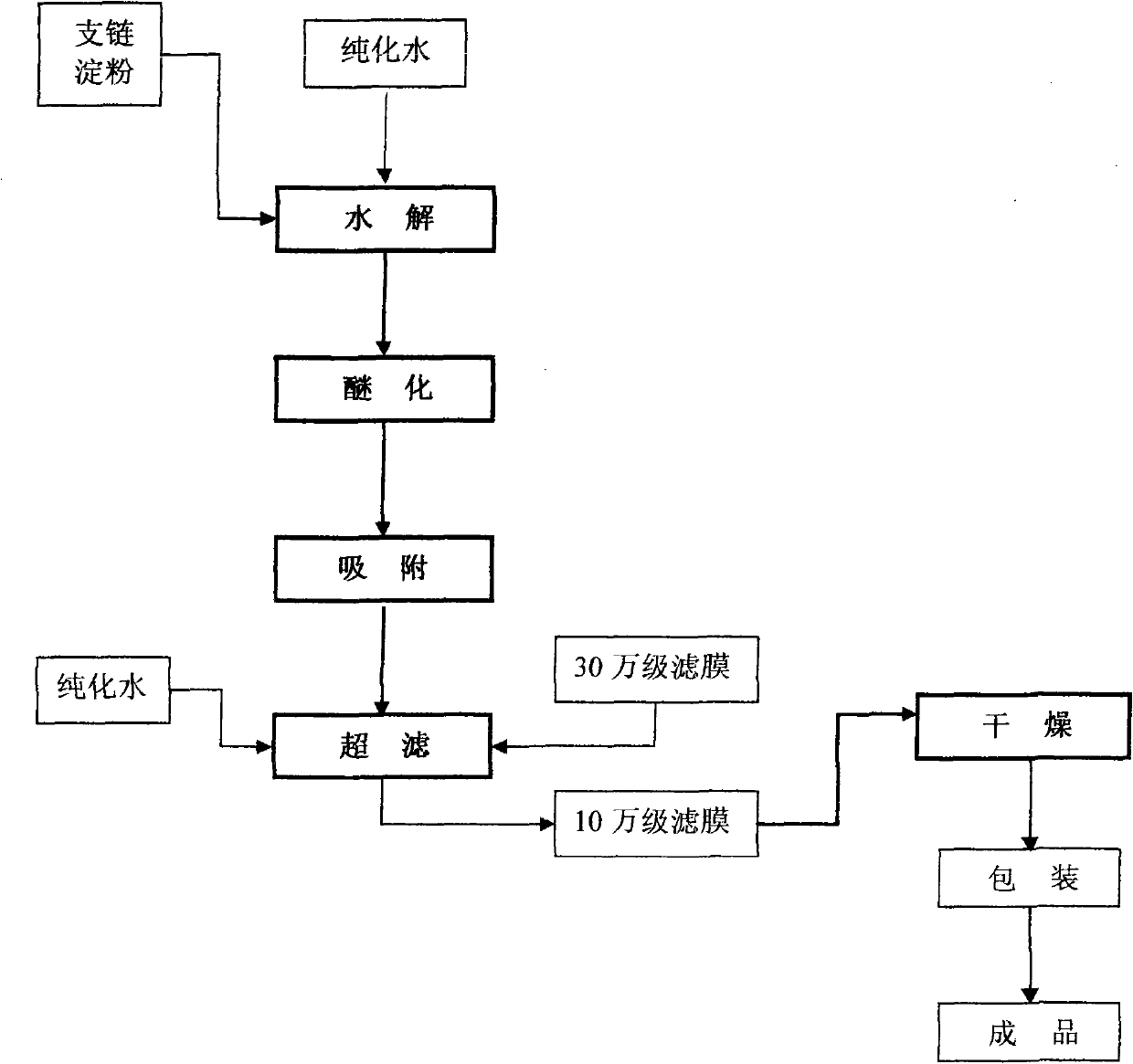
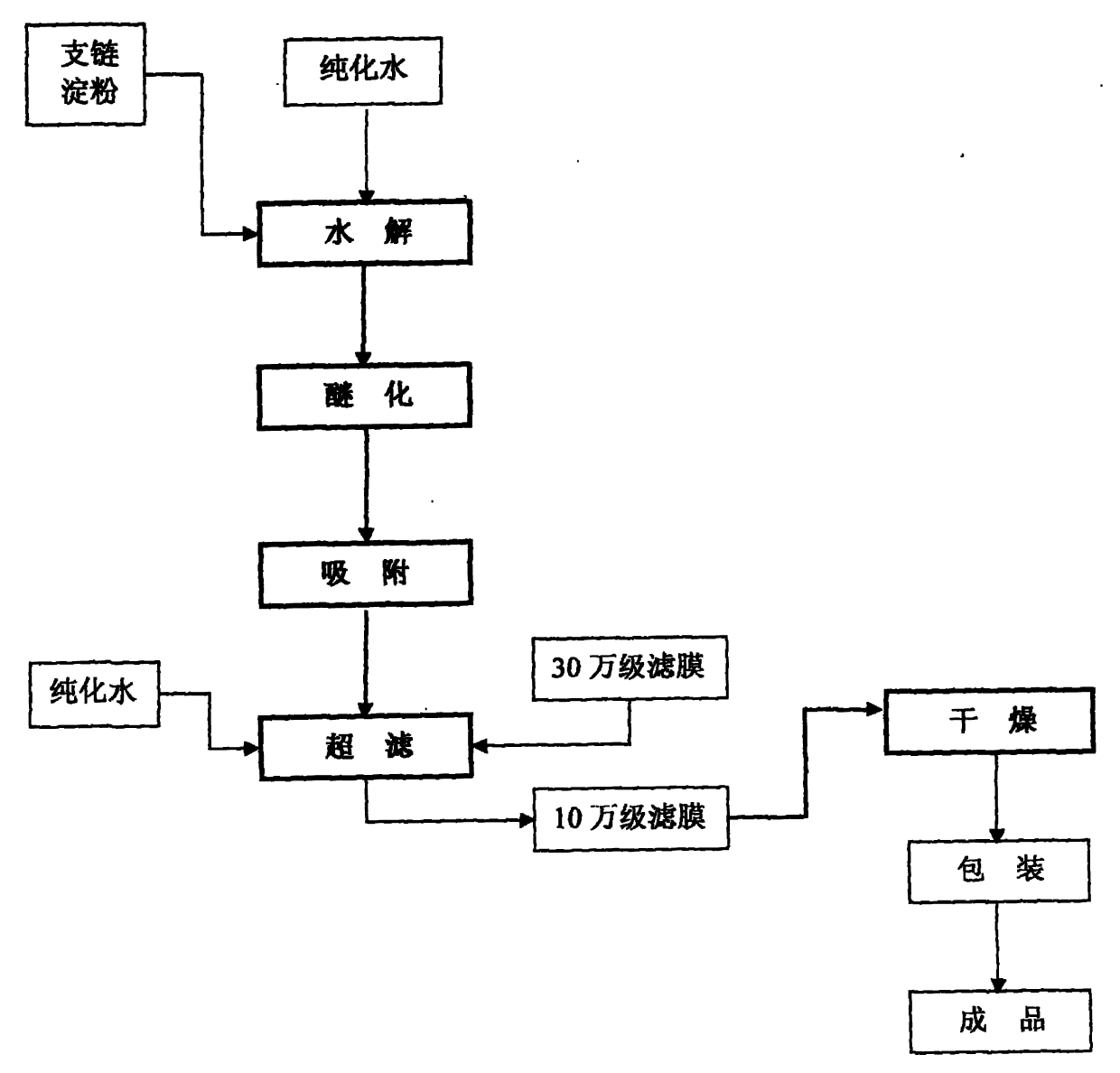
[0009] Accompanying drawing is the schematic diagram that improves the preparation method of medium molecular weight hydroxyethyl starch quality according to the present invention, as shown in the figure, concrete implementation method and technique are as follows:
[0010] 1. Hydrolysis
[0011] 2. Etherification
[0012] 3. Adsorption
[0013] 4. Ultrafiltration uses a filter membrane with a molecular weight cut-off (KD) of 300,000 to remove large molecular weight endotoxin associations, and then uses a filter membrane with a molecular weight cut-off (KD) of 100,000 to screen the final molecular weight to complete the feed liquid refined.
[0014] 5. Drying to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com