Lornoxicam tromethamine eutectic crystal
A technology of lornoxicam tromethamine and lornoxicam, applied in the field of medicine, can solve the problems of affecting dissolution rate and bioavailability, low solubility of lornoxicam and the like
Inactive Publication Date: 2011-06-15
CHINA PHARM UNIV
View PDF5 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Lornoxicam has very little solubility in water, thus affecting its dissolution rate and bioavailability in water
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
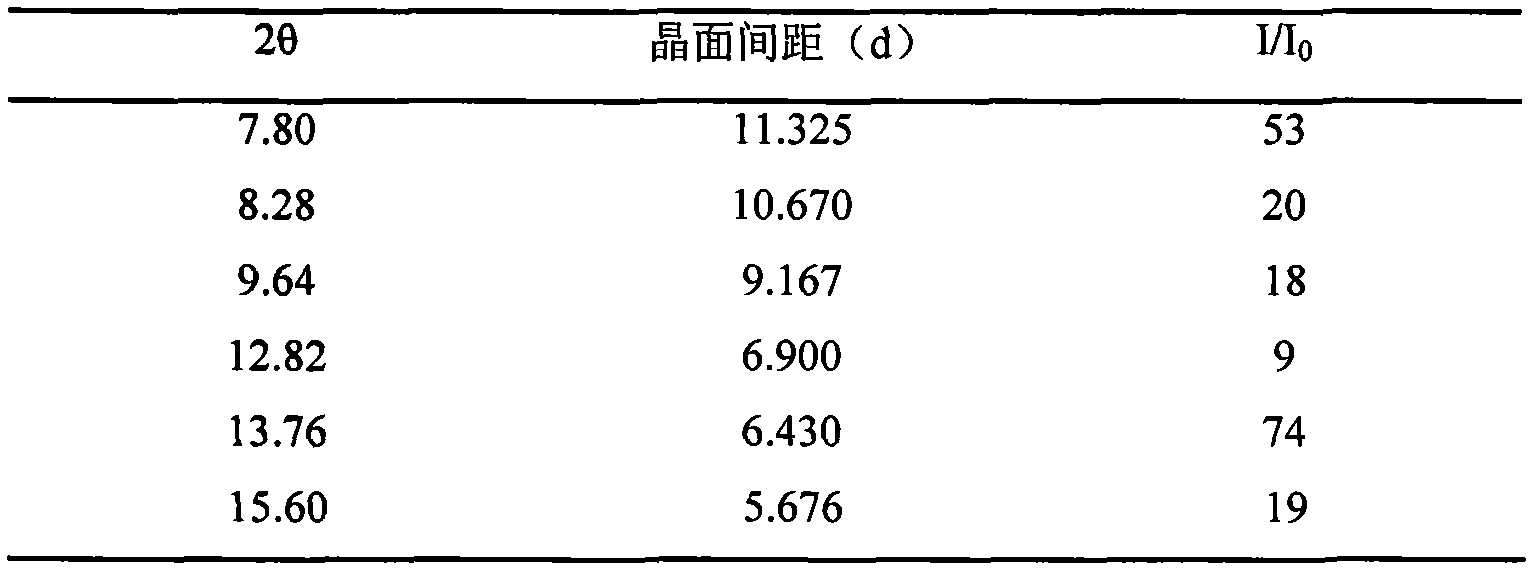
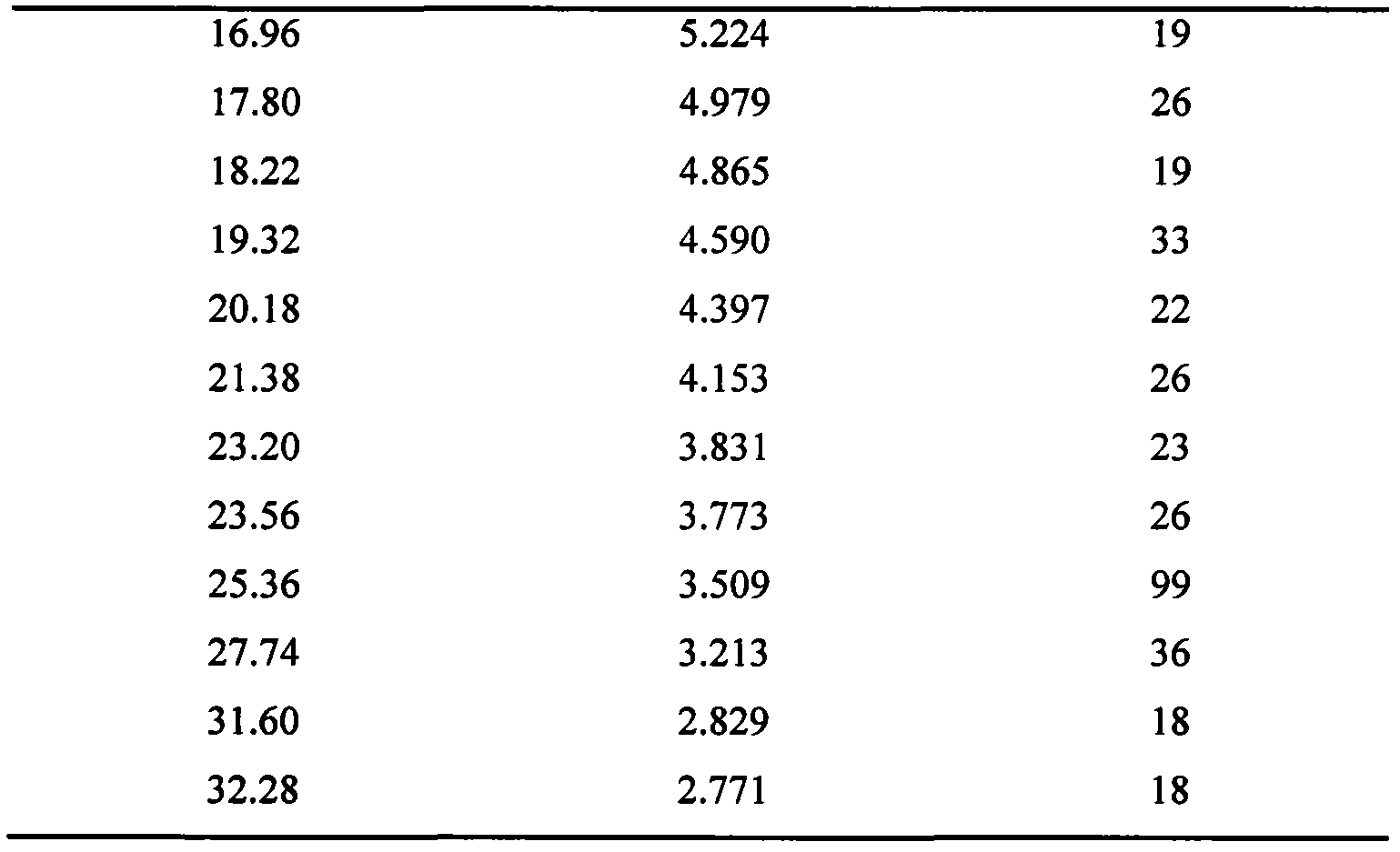
The invention relates to a lornoxicam tromethamine eutectic crystal formed by combining lornoxicam and tromethamine. By Cu-K alpha radiation, characteristic peaks are presented at the positions of 7.80, 8.28, 9.64, 12.82, 13.76, 15.60, 16.96, 17.80, 18.22, 19.32, 20.18, 21.38, 23.20, 23.56, 25.36, 27.74, 31.60 and 32.28 of X-ray powder diffraction spectrum which is represented in a degree of 2 theta; absorption peaks are presented at the positions of about 3352, 3294, 3103, 2937, 2887, 1627, 1573, 1533, 1496, 1473, 1433, 1392, 1332, 1236, 1188, 1155, 1045, 887, 821, 777, 634, 588, 551,522 and 462 cm<-1> wavelengths of infrared absorption spectrum obtained by KBr tebletting; and heat absorption transformation of differential scanning caborimetry (DSC) is mainly generated at the temperature of about 55.9 DEG C. The lornoxicam tromethamine eutectic crystal disclosed by the invention is different from the existing commercial lornoxicam powder in the aspects of X-ray diffraction, DSC, infrared spectrum and melting point, thus the crystal form of the lornoxicam tromethamine eutectic crystal is a crystal form which is completely different from the lornoxicam prepared by the prior art. Compared with the lornoxicam physical mixture of equivalent tromethamine, the digestion speed and degree of the lornoxicam tromethamine eutectic crystal are obviously improved.
Description
Lornoxicam Tromethamine Cocrystal technical field The invention belongs to the technical field of medicine, and in particular relates to lornoxicam tromethamine co-crystal and a preparation method thereof. Background technique Lornoxicam, 6-Chloro-4-hydroxy-2-methyl-3-(2-pyridinecarbamoyl)-2H-thieno[2,3-e]-1,2-thiazine-1 , 1-dioxide (Lornoxicam), a thiazide derivative, is a non-steroidal anti-inflammatory analgesic, clinically used to treat acute and chronic pain caused by inflammation, such as postoperative pain, arthritis, rheumatism , soft tissue injury, etc. Lornoxicam inhibits the synthesis of prostaglandins by inhibiting the activity of arachidonic acid cyclooxygenase, so it has strong analgesic and anti-inflammatory effects. Lornoxicam has very little solubility in water, thus affecting its dissolution rate and bioavailability in water. Patent CN101185868 discloses a process for preparing lornoxicam fine particles using supercritical fluid crystallization techno...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D513/04C07C215/10C07C213/08
Inventor 张建军高缘谭欣
Owner CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com