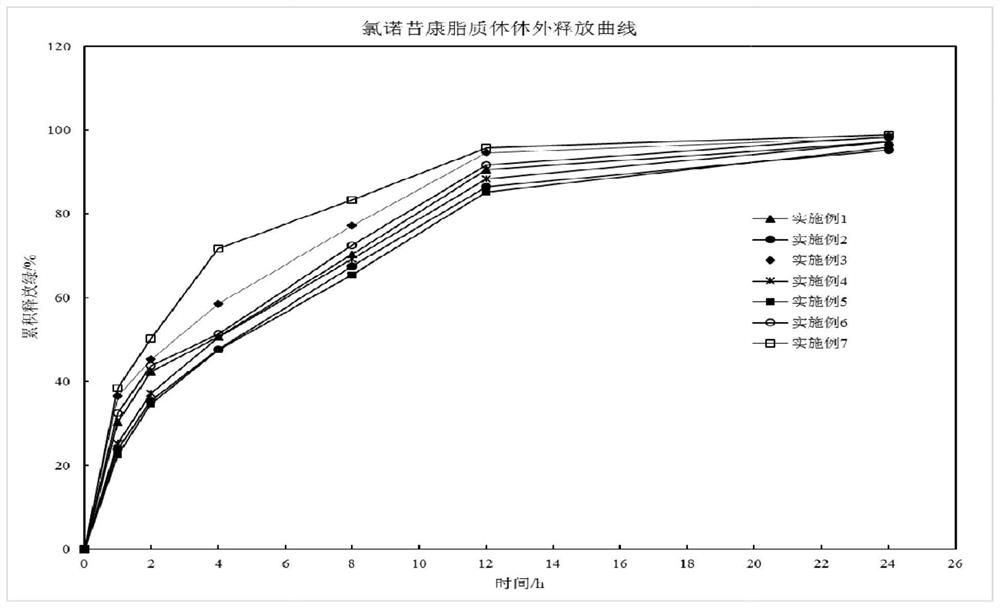
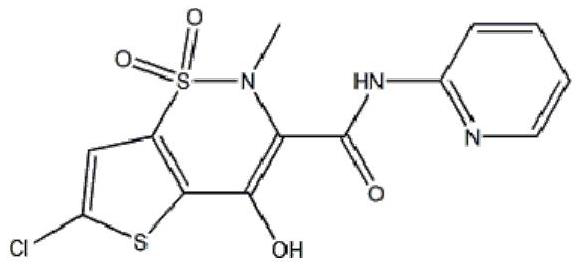
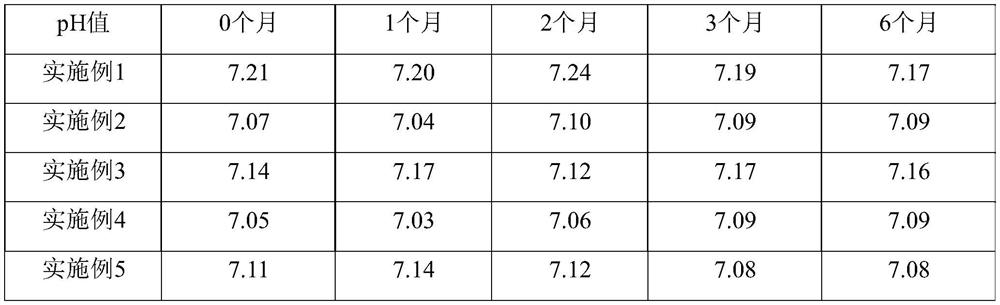
Lornoxicam liposome for injection
A technology of lornoxicam fat and lornoxicam, which is applied in the field of lornoxicam liposome for injection, can solve the problems of poor compliance and high pH of lornoxicam powder injection, and achieve mild pH value and stability And the effect of good reproducibility and long-term sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment provides a lornoxicam liposome for injection, the components of the lornoxicam liposome for injection include 5 parts by weight of lornoxicam, 8 parts of hydrogenated soybean phosphatidylcholine parts, cholesterol 2 parts, PEG400 3 parts, phosphate buffer 5 parts, trehalose 3 parts.
[0045] Its preparation method is:
[0046] (1) Dissolving lornoxicam, hydrogenated soybean phosphatidylcholine, cholesterol, and PEG400 in chloroform at 50° C. according to the formula to obtain an oil phase solution.
[0047] (2) According to the formula, the phosphate buffer and water are mixed and dissolved at 50° C. to obtain an aqueous phase solution.
[0048] (3) The oil phase solution in step (1) is placed in a vacuum evaporator to remove chloroform.
[0049] (4) mixing the aqueous phase solution in step (2) with the oil phase in step (3), and placing it under a high-speed shearing machine for emulsification.
[0050] (5) placing the emulsified sample in step (4) ...
Embodiment 2
[0053] This embodiment provides a lornoxicam liposome for injection. The components of the lornoxicam liposome for injection include, in parts by weight, 1 part of lornoxicam, 5 parts of refined egg yolk lecithin, 1 part cholesterol, 3 parts poloxamer, 5 parts phosphate buffer, 5 parts mannitol.
[0054] Its preparation method is:
[0055] (1) Dissolving lornoxicam, refined egg yolk lecithin, cholesterol, and poloxamer in a chloroform / ethanol mixed solvent at 70° C. to obtain an oil phase solution according to the formula.
[0056] (2) According to the formula, the buffer and water are mixed and dissolved at 70° C. to obtain an aqueous phase solution.
[0057] (3) The oil phase solution in step (1) is placed in a rotary evaporator to remove the chloroform / ethanol mixed solvent.
[0058] (4) Mix the aqueous phase solution in step (2) with the oil phase in step (3), and place it under ultrasonic to emulsify.
[0059] (5) Place the emulsified sample in step (4) in a high press...
Embodiment 3
[0062] This embodiment provides a lornoxicam liposome for injection, the components of the lornoxicam liposome include 2 parts by weight of lornoxicam and 10 parts of dipalmitate phosphatidylcholine , 2 parts cholesterol, 1 part poloxamer, 5 parts phosphate buffer, and 5 parts sucrose.
[0063] Its preparation method is:
[0064] (1) Dissolving lornoxicam, dipalmitate phosphatidylcholine, cholesterol, and poloxamer in chloroform at 50° C. according to the formula to obtain an oil phase solution.
[0065] (2) According to the formula, the buffer and water are mixed and dissolved at 50° C. to obtain an aqueous phase solution.
[0066] (3) The oil phase solution in step (1) is placed in a vacuum evaporator to remove chloroform.
[0067] (4) mixing the aqueous phase solution in step (2) with the oil phase in step (3), and placing it under a high-speed shearing machine for emulsification.
[0068] (5) Place the emulsified sample in step (4) in a nitrogen extruder to homogenize t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com