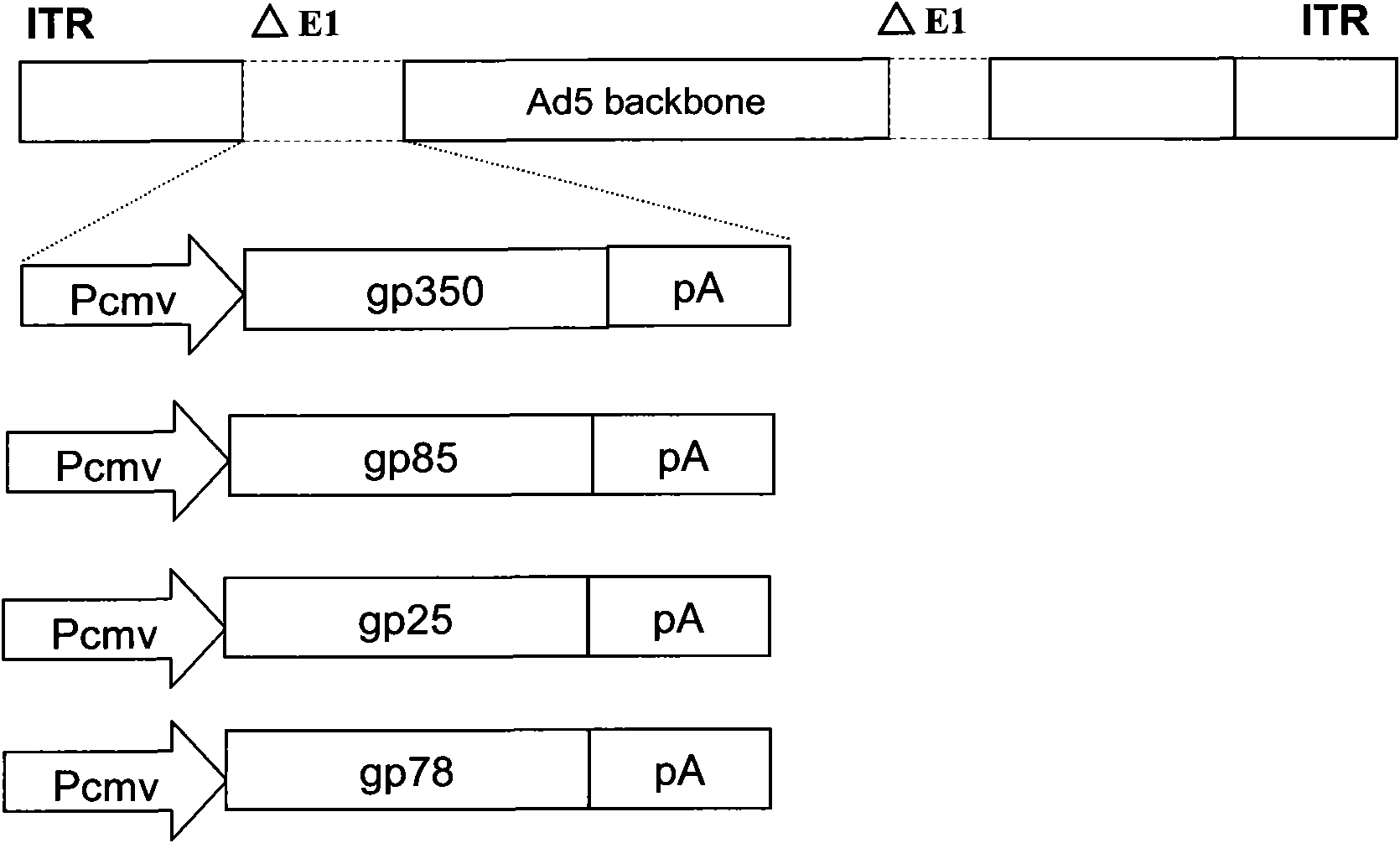
Recombinant adenovirus vaccine containing EB (Epstein-Barr) virus membrane protein gp350/220, gp85, gp78 or gp25 gene
A technology of recombinant adenovirus and EB virus, applied in the field of or gp2, can solve the problems of difficulty in scale, limited production of natural gp350, etc., and achieve the effects of various and convenient use forms, prevention of virus infection, and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0040] In this application, unless otherwise specified, the implementation of the present invention uses conventional techniques of molecular biology, molecular biology, recombinant DNA and immunology, which are all conventional techniques mastered by those skilled in the art. These techniques are described in detail in the following documents: Molecular Cloning: Alaboratory Manual, 2nd Edition (1989), edited by Sambrook et al.; Nucleic Acid Hybridization (edited by B.D.Hames and S.J. Higgin, 1984); Immnochemical Methods in Cell And Molecular Biology (Academic Press , London).
[0041] Materials and methods:
[0042] Epstein-Barr virus strain GD1 derived from nasopharyngeal carcinoma tissue used in the present invention (established by our laboratory, JOURNALOF VIROLOGY, Dec.2005, p.15323-15330);
[0043] Adenovirus shuttle plasmid pENTR2B, adenovirus backbone plasmid, Escherichia coli host cell JM109 and adenovirus packaging cells were purchased from INVITROGEN, USA.
[004...
Embodiment 1
[0049] Example 1: Construction of a recombinant adenoviral vector containing Epstein-Barr virus glycoprotein gp350 / 220 gene sequence
[0050] 1.1 Construction of the shuttle plasmid
[0051] According to the EBV membrane protein gp350 / 220 gene sequence published by the gene bank (gene bank query number AY961628.3), the PCR primers were designed as follows:
[0052] Upstream primer P1: TTGAGTCGACACCATGGAGGCAGCCTTGCTTGT;
[0053] Downstream primer P2: TCAGGAGAGGTTTGAGAATCTGG;
[0054] The PCR reaction system is: deionized water ddH2O 16.3uL, PCR Buffer2.5uL, upstream primer P1: 0.5uL, downstream primer P2: 0.5uL, dNTP4.0uL, Taq enzyme: 0.2ul, CD1 template 1uL, a total of 25U1. Use ddH2O instead of the template as a negative control.
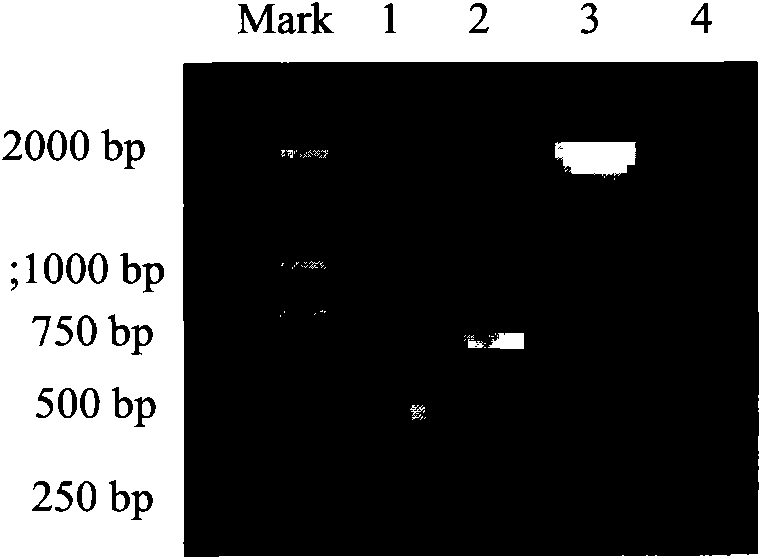
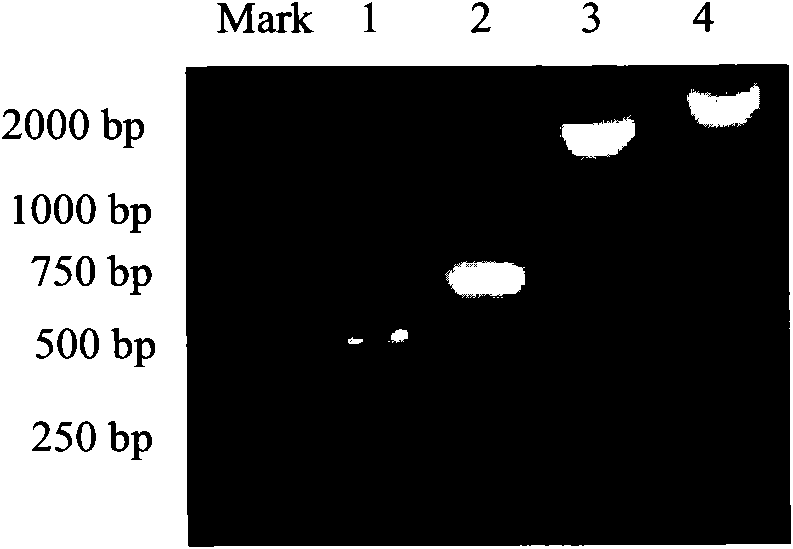
[0055] The PCR reaction conditions are: 95°C, 5min denaturation; 95°C, 30s, 55°C, 45s, 72°C, 1min, a total of 30 cycles, 72°C, 7min. Check the PCR products on an agarose gel, see figure 1 .
[0056] The PCR product was recovered by electropho...
Embodiment 2
[0092] 2.1 Construction of the shuttle plasmid
[0093] According to the EBV membrane protein gp85 gene sequence published by the gene bank (gene bank query number GPAY96162.8), the PCR primers were designed as follows:
[0094] Upstream primer P3: ACCGTCGACATGGGCCAGTTGCTCTGTGTTTTTTGC;
[0095] Downstream primer P4: TCAGTGTGCCTCTTTCTTCATACAG;
[0096] The PCR reaction system is: deionized water ddH2O 16.3uL, PCR Buffer 2.5uL, upstream primer PI: 0.5uL, downstream primer P2: 0.5uL, dNTP 4.0uL, Taq enzyme: 0.2uL, CD1 template 1uL, a total of 25uL. Use ddH2O instead of the template as a negative control.
[0097] The PCR reaction conditions are: 95°C, 5min denaturation; 95°C, 30s, 55°C, 45s, 72°C, 1min, a total of 30 cycles, 72°C, 7min. The length of the fragment amplified by PCR is 2200bp; (please supplement) see figure 1
[0098] The PCR product was recovered by electrophoresis using an agarose gel method to obtain PCR product 1. For recovery steps, see the manual of Mine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com